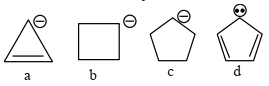
કાર્બએનાયન ની સ્થિરતા નો સાચો ક્રમ શોધો.

JEE MAIN 2024, Medium
d
As we know compound (\(d\)) is aromatic and the compound (\(a\)) is anti-aromatic. Hence compound
As we know compound (\(d\)) is aromatic and the compound (\(a\)) is anti-aromatic. Hence compound
(\(d\))is most stable and compound (\(a\)) is least stable among these in compound (\(b\)) and (\(c\)) carbon atom of that positive charge is \(\mathrm{sp}^3\) hybridised they on the basis of angle strain theory compound (\(c\)) is more stable than compound (\(b\)).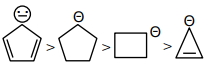
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1વિસ્થાપકોની $-I$ અસરના સંદર્ભમાં નીચે આપેલામાંથી કયું સાચું છે ? ($R =$ આલ્કાઇલ સમૂહ)View Solution
- 2સૌથી વધુ $K_a$ સાથે એસિડ પસંદ કરોView Solution
- 3નીચેના માટે ઈલેકટ્રોન અનુરાગી માટે ઘટતો પ્રક્રિયાશીલતા ક્રમ કયો છે ?View Solution
$(1)$ બેન્ઝીન $(2)$ ટોલ્યુઈન $(3)$ ક્લોરો બેન્ઝિન $(4)$ ફિનોલ
- 4View Solutionનીચેનામાંથી કયા સમઘટકીય બંધારણમાં સૌથી ઓછી ઉર્જા છે?
- 5View Solutionએસિડિક સ્વભાવનો ક્રમ શું હશે ?
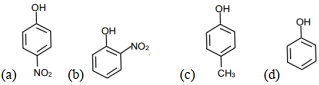
- 6કાર્બોકેટાયનને તેમની ઘટતી સ્થિરતાના ક્રમમાં ગોઠવો.View Solution
$(1) H_3C - C = C⊝\,(2) H - C = C⊝ \,(3) $ ${H_3}C\,\, - \,\,\mathop C\limits^\Theta {H_2}$
- 7View Solutionનીપજની સ્થિરતાની સમજના આધારે, જ્યારે નીચેનો ડાયએનાયન એસિડના સમકક્ષ સાથે પ્રક્રિયા આપે ત્યારે રચાયેલી નીપજ ની આગાહી કરો
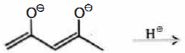
- 8View Solutionકાર્બોકેશાયનો (કાર્બોકિટાયનો)નો સાચો સ્થિરતા ક્રમ શોધો.
- 9$NH_3, CH_2NH_2$ અને $(CH_3)_2NH$ બેઈઝ માટે વધતા બેઝિક સ્વભાવનો સાચો ક્રમ કયો છે ?View Solution
- 10View Solutionનીચેનામાંથી સૌથી વધુ ધ્રુવીય છે
