કેલ્શિયમ ઑક્સાઈડની હાજરીમાં, સોડિયમ ઈથેનોએટ સાથે સોડિયમ હાઈડ્રોક્સાઈડને ગરમ કરતાં મળતા કાર્બનિક સંયોજનના બે $moles$નું વજન $(g)$ શોધો.
NEET 2023, Diffcult
c
This reaction is called soda lime decarboxylation
This reaction is called soda lime decarboxylation
Molar mass of $CH _4=16\,g / mol$
Weight of 2 moles of $CH _4=16 \times 2$
$=32\,g$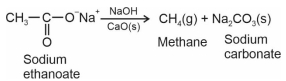
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1Alkene $(A)$ will beView Solution
$\mathop A\limits_{(alkene)} \xrightarrow[{{H_2}O}]{{RC{O_3}H}}$ રેસેમિક મિશ્રણ
$\mathop A\limits_{(alkene)} \xrightarrow{{Cold\,dil.KMn{O_4}}}$ મેસો સંયોજન
- 2નીચેની શૃંખલામાં $Z$ શું હશે?View Solution
$C{H_2} = C{H_2}\xrightarrow{{HBr}}X\xrightarrow{{{\text{Hydrolysis}}}}Y\mathop {\xrightarrow{{N{a_2}C{O_3}}}}\limits_{{I_2}{\text{ excess}}} Z$
- 3પેરોક્સાઇડની હાજરીમાં, $HCl$ અને $HI$ એ આલ્કીન સાથે પ્રતિમાર્કોનિકોવ નિયમ મુજબ યોગશીલ પ્રક્રિયા આપતા તથી, કારણ કે .........View Solution
- 4View Solutionપ્રક્રિયામાં ઉદભવતી નીપજ કઈ છે ?
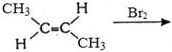
- 5View Solutionઆપેલ પ્રક્રિયામાં નીપજ કઈ છે ?
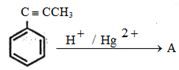
- 6View Solutionઉપરોક્ત પ્રકિયા માટે યોગ્ય પ્રકીયક કયો છે ?
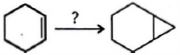
- 7કઈ પ્રક્રિયા દ્વારા $1$-બ્યુટીને એ બ્યુટેનમાં રૂપાંતર પામે છે ?View Solution
- 8View Solutionનીચેનામાંથી કોણ એસીડીક હાઇડ્રોજન ધરાવે છે.
- 9View Solutionકયો અણુ દ્વિ - ધ્રુવ ચાકમાત્રા ધરાવે છે ?
- 10પ્રક્રિયા $C{H_2} = C{H_2} + {H_2}\mathop {\xrightarrow{{Ni}}}\limits_{250 - {{300}\,^o}C} C{H_3} - C{H_3}$ને કહેવાય છે..... .View Solution
