ખોટું વિધાન કયું છે ?
JEE MAIN 2020, Medium
c
Option $1)$ Manganate $\Rightarrow MnO _{4}^{2-}$
Option $1)$ Manganate $\Rightarrow MnO _{4}^{2-}$
Permanganate $\Rightarrow MnO _{4}^{-}$
$2 \times 2 P _{\pi}-3 d _{\pi}$
$1 \times 2 P _{\pi}-4 P _{\pi}$
$(2)$ $MnO _{4}^{2-} \Rightarrow$ green
$MnO _{\stackrel -4} \Rightarrow$ purple/violet
$(3)$ Manganate contains $1$ unpaired electron hence it is paramagnetic
where as permanganetic contains no unpaired electrons hence it is diamagnetic.
$(4)$ Both have d's hybridisation hence both have tetrahedral geometry.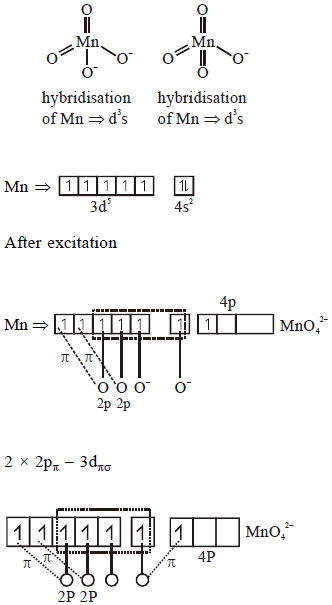
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચે દર્શાવેલ આયનોની જોડ પૈકી કઇ જોડનાં બંને આયનો જલીય દ્રાવણમાં રંગીન હશે (પરમાણુક્રમાંક $ : Se = 21, Ti = 22, Co = 27, Ni = 28, Cu = 29)$View Solution
- 2નિઓબિયમ માટે ઈલેક્ટ્રોન સંરચના શોધો. [નિઓબિયમ માટે પરમાણુક્રમાંક $60$]View Solution
- 3$\mathrm{NaCl}$ ની સાંદ્ર $\mathrm{H}_2 \mathrm{SO}_4$ અને $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ સાથે પ્રક્રિયા કરતા લાલાશ પડતો ધુમાડો ($B$) આપે છે, કે જે $\mathrm{NaOH}$ સાથે પ્રક્રિયા કરતાં પીળું દ્રાવણ ($C$) આપે છે. ($B$) અને ($C$) અનુક્રમે શોધો.View Solution
- 4નીચેનામાંથી ક્યો ઘન ક્ષાર ઘન $K_2Cr_2O_7$ અને સાંદ્ર. $H_2SO_4$ સાથે ગરમ કરવા પર નારંગી લાલ વરાળ વિકસિત થાય છે જે જલીય $NaOH$ દ્રાવણ પીળું છે?View Solution
- 5........ ના ફેરફારના કારણે $\mathrm{Mn}^{3+} / \mathrm{Mn}^{2+}$ યુગ્મ (couple) માટે $\mathrm{E}^{\circ}$ મૂલ્ય એ $\mathrm{Cr}^{3+} / \mathrm{Cr}^{2+}$ અથવા $\mathrm{Fe}^{3+} / \mathrm{Fe}^{2 *}$ કરતાં વધારે ધન (Positive) છે.View Solution
- 6View Solutionચતુર્થ સંક્રાતિ શ્રેણીમાં નીચેના પૈકી કયા તત્વોને સમાવેશ થાય છે
- 7. . . . . . .માં સંતુલન $\mathrm{Cr}_2 \mathrm{O}_7^{2-} \rightleftharpoons 2 \mathrm{CrO}_4^{2-}$જમણી તરફ ખસે છે :View Solution
- 8$Cr $ ની ઇલેકટ્રોન રચના નીચેના પૈકી કઇ છેView Solution
- 9મેગેનીઝ $(VI$) એસિડિક દ્રાવણમાં વિષમીકરણ થવા માટે ક્ષમતા ધરાવે છે.એસિડિક માધ્યમમાં બનતા બે આયનોની ઓકિસડેશન અવસ્થાઓનો તફાવત $\dots\dots$છે.View Solution
- 10ચાર ક્રમિક સંક્રમણ તત્વો $(Cr, \,Mn,\, Fe$ અને $Co),$ માટે $+ 2$ ઓક્સિડેશન અવસ્થાની સ્થિરતા નીચેના કયા ક્રમમાં હશે?View Solution
