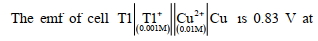$Pt _{( s )}\left| H _2( g , 1\,atm )\right| H ^{+}( aq , 1 M )|| Fe ^{3+}( aq ), Fe ^{2+}( aq ) \mid Pt ( s )$
$298\,K$ પર જયારે કોષ નો પોટેન્શિયલ $0.712\,V$ હોય તો $\left[ Fe ^{2+}\right] /\left[ Fe ^{3+}\right]$ નો ગુણોત્તર $.......$ છે.
આપેલ:$Fe ^{3+}+ e ^{-}= Fe ^{2+}, E ^{\circ} Fe ^{3+}, Fe ^{2+} \mid Pt =0.771$
$\frac{2.303 RT }{ F }=0.06\,V$
\(Pt _{( s )}\left| H _2( g , 1 atm )\right| H ^{+}( aq , 1 M ) \| Fe ^{3+}( aq ), Fe ^{2+}( aq )| Pt |( s )\)
\(\text { at anode } H _2 \longrightarrow 2 H ^{+}+2 e ^{-}\)
\(\text {At cathode } Fe _{ aq }^{3+}+ e ^{-} \longrightarrow Fe _{ aq }^{2+}\)
\(E ^{\circ}= E _{ H _2 / H ^{+}}^{\circ}+ E _{ Fe ^{3+} Fe ^{2+}}^{\circ}=0 \cdot 771\,V\)
\(E = E ^{\circ}-\frac{0 \cdot 06}{1} \log \frac{ Fe ^{2+}}{ Fe ^{3+}}\)
\(0 \cdot 712=(0+0 \cdot 771)-\frac{0 \cdot 06}{1} \log \frac{ Fe ^{2+}}{ Fe ^{3+}}\)
\(\log \frac{ Fe ^{2+}}{ Fe ^{3+}}=\frac{0 \cdot 059}{0 \cdot 06} \approx 1\)
\(\frac{ Fe ^{2+}}{ Fe ^{3+}}=10\)
Download our appand get started for free
Similar Questions
- 1$2 $ મોલ $Na^{+}$ ના સંપૂર્ણ વિદ્યુત વિભાજન માટે જરૂરી કુલ ભાર..... (કુલમ્બ).View Solution
- 2કોષ પ્રક્રિયા $Zn + Cu^{2+} \rightarrow Cu + Zn^{2+}$ માટે પ્રમાણિત $EMF$ નું મૂલ્ય $25°\,C$ તાપમાને $1.10 \,V$ છે. જો $0.1\, M\, Cu^{2+}$ અને $0.1\, M\, Zn^{2+}$ ના દ્રાવણો વાપરવામાં આવે, તો $E.M.F$ નું ........... $V$ થાય.View Solution
- 3ઔદ્યોગિક ઉત્પાદનમાં વિદ્યુત વિભાજનથી બે કલાકમાં $40$ કિ.ગ્રા. કેલ્શિયમ મળે છે. બે કલાક માટે આટલો જ પ્રવાહ પસાર કરતા કેટલા ............ $\mathrm{kg}$ એલ્યુમિનિયમ ઉત્પન્ન થાય? ($Ca$ નો પ.ભા.$= 40,\, Al = 27$)View Solution
- 4એસિડીક પાણીમાં $0.4$ એમ્પિયર પ્રવાહ $30$ મિનિટ માટે પસાર કરવામાં આવે તો $STP$ એ હાઈડ્રોજનના કદની ગણતરી ............... લિટરમાં કરો.View Solution
- 5વિધુતરસાયણિક કોષના $emf$ માટે નીચેના સંબંધ વિચારો.View Solution
$(i)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $-$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(ii)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $+$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(iii)$ કોષનો $EMF$ $=$ (એનોડનો રીડકશન પોટેન્શિયલ) $+$ (કેથોડનો રીડકશન પોટેન્શિયલ)
$(iv)$ કોષનો $EMF$ $=$ (એનોડનો ઓક્સિડેશન પોટેન્શિયલ) $-$ (કેથોડનો ઓક્સિડેશન પોટેન્શિયલ)
નીચેના પૈકી ક્યા સંબંધો સાચા છે ?
- 6$Pt ( s ) H _2( g )(1 bar )\left| H ^{+}( aq )(1 M )\right|\left| M ^{3+}( aq ), M ^{+}( aq )\right| Pt ( s )$View Solution
$298\,K$ પર જ્યારે $\frac{\left[M^*(a q)\right]}{\left[M^{3 *}(a q)\right]}=10^a$ હોય ત્યારે આપેલ કોષ નો $E_{\text {cell }}$ એ $0.1115\,V$ છે. $a$ નું મૂલ્ય $............$ છે.આપેલ : $E _{ M }^\theta{ }^{3+} M ^{+}=0.2\,V$
$\frac{2.303\,R T}{F}=0.059\,V$
- 7$1\, M$ એસિટિક ઍસિડનો અવરોધ $250$ ઓહ્મ છે. વાહકતાકોષનો કોષ-અચળાંક $1.15 $ સેમી$^{-1}$ છે, તો $1\, N$ એસિટિક ઍસિડની તુલ્યવાહકતા (ઓહમ$^{-1}$ સેમી$^{2}$ તુલ્ય$^{-1}$) કેટલી થશે ?View Solution
- 8View Solutionવિદ્યુતવિભાજન દરમિયાન કૅથોડ પાસે વીજભારવિહીન બનતો ઘટક ...... છે.
- 9$STP$ એ $5600$ મિલી હાઈડ્રોજનનું સ્થાનાતારણ માટે વપરાયેલ પ્રવાહનો જથ્થો જેટલું મૂલ્ય કેટલા ............. ગ્રામ સિલ્વરના (તુ.ભા.$=108$) સ્થાનાતરણ માટે વપરાય છે?View Solution
- 10$298 \mathrm{~K}$. પર કોષ (image) ના વડે તેને વધારી શકાય છે.View Solution