$\left[{Co}\left({CN}_{6}\right)\right]^{4-}$ સંકીર્ણમાં ફકત સ્પિન ચુંબકીય ચાકમાત્રાનું મૂલ્ય $ ...... \, BM$ [પરમાણ્વીય ક્રમાંક ${Co}=27$ ]
JEE MAIN 2021, Diffcult
b
${\left[{CO}({CN})_{6}\right]^{4-}}$
${\left[{CO}({CN})_{6}\right]^{4-}}$
${x}+6 \times(-1)=-4$
${x}=+2$
${Co}^{2+}:[{Ar}] 3 {~d}^{7}$
and ${CN}^{-}$is a strong field ligand which can pair electron of central atom.
It has one unpaired electron ( $n$ ) in $4d-$subshell.So spin only magnetic moment $(\mu)=\sqrt{n(n+2)}\, B . M$
where ${n}=$ number of unpaired electrons.
$\mu=\sqrt{3} \,{~B} . {M} \quad \mu=1.73\, {BM}$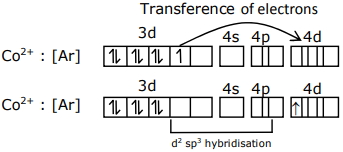
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1આયર્ન $(III)$ હેકઝાસાયનોફેરેટ $(II)$ નુ બંધારણ .......... થશે.View Solution
- 2$[Pd^{2+} (NH_2 -CH(CH_3 )-CO^-_2)_2]$ માટે ભૌમિતિક સમઘટક કેટલા શક્ય છે?View Solution
- 3$[CoCl(NO_2)(en)_2]Cl$ નું $IUPAC$ નામ.....View Solution
- 4$[VO(acac)_2]$ એ સવર્ગ સંયોજન..... નુ અણુસૂત્ર છે.View Solution
- 5નીચે આપેલા માંથી કયા માં $AgCl$ ની દ્રાવ્યતા સૌથી મહત્તમ (વધુ) થશે ?View Solution
- 6$\left[ Mn _{2}( CO )_{10}\right]$ માં સેતુમય $CO$ લિગેંડ્સ ની સંખ્યા .......... છે.View Solution
- 7નીચેનામાંથી એક સંરૂપણ માટેની સ્પીન ચુંબકીય ચાકમાત્રા મૂલ્ય $2.84\ BM$ છે તો સાચું કયું છે?View Solution
- 8નીચેના પૈકી કયા સંયોજન/સંયોજનો ભૌમિતિક સમઘટકતા દર્શાવે છે?View Solution
$(i) [pt\ (en)\ Cl_2]$
$(ii)\ [pt (en)_2]\ Cl_2$
$ (iii)\ [pt (en)_2 Cl_2]\ Cl_2$
$(iv)\ [pt (CH_3)_2 Cl_2]$
- 9નીચેનામાંથી કયું સંકીર્ણ સમતલીય સમચોરસ રચના ધરાવશે ?View Solution
(પ.ક્ર. : $Fe=26, Co= 27, Ni =28, Pt= 78$)
- 10View Solutionનીચેના પૈકી ક્યુ સૌથી પ્રબળ ક્ષેત્ર લિગેન્ડ છે ?
