$(1)$ $C{H_3} - \mathop {CH}\limits^ \bullet C{H_3}$
$(2)$ (ચિત્ર.)
$(3)$ $\mathop {C{H_2}}\limits^ \bullet - CH{(C{H_3})_2}$
$(4)$ $\mathop {C{H_2}}\limits^ \bullet - C{H_3}$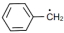
$(1)$ $C{H_3} - \mathop {CH}\limits^ \bullet C{H_3}$
$(2)$ (fig.)
$(3)$ $\mathop {C{H_2}}\limits^ \bullet - CH{(C{H_3})_2}$
$(4)$ $\mathop {C{H_2}}\limits^ \bullet - C{H_3}$
The stability of free radicals is mostly affected by the factors like resonance, hyperconjugation and inductive effect. In compound $ (II)$, there is high resonance effect due to which it is highly stable than other three compounds. The effect of hyperconjugation is directly proportional to the number alpha hydrogen. So, compound $I$ has six alpha hydrogen atoms, compound $III $ has one alpha hydrogen atom and compound $IV$ has three alpha hydrogen atoms. Therefore, the order is $II > I > IV > III.$
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાનથી કયું બેંઝિન નું વિસ્થાપન ઇલેક્ટ્રોનુરાગી વિસ્થાપન માં ઓર્થો-પેરા અને કેંદ્રાનુરાગી વિસ્થાપન માં ઓર્થો-પેરા માં છે
- 2$C{H_3}C{H_2}I + KO{H_{(aq)}} \to C{H_3}C{H_2}OH + KI$ પ્રક્રિયાને નીચે દર્શાવેલા પૈકી શેમાં વર્ગીકૃત કરી શકાય છે ?View Solution
- 3View Solutionઉપરોક્ત સંયોજનમાં હાજર મોટાભાગના એસિડિક હાઇડ્રોજનને ઓળખો
- 4કાર્બાનિયનની સ્થિરતાનો ઘટતો ક્રમ નીચેનામાંથી કયો છે ?View Solution
$(1)\,\,{{(C{{H}_{3}})}_{3}}\bar{\ddot{C}}$
$(2)\,\,{{(C{{H}_{3}})}_{2}}\bar{\ddot{C}}$
$\,(3)\,\,C{{H}_{3}}\bar{\ddot{C}}{{H}_{2}}$
$(4)\,\,{{C}_{6}}{{H}_{5}}\bar{\ddot{C}}{{H}_{2}}$
- 5View Solutionએસિડિક પ્રબળતામાં વધારો કરવાના ક્રમ આપો
- 6View Solutionનીચેનામાંથી કયો સૌથી પ્રબળ બેઇઝ છે ?
- 7View Solutionનીચેનામાંથી મુકતમુલક નો સ્થાયિતાનો વધતો ક્રમ કયો છે?
- 8View Solutionઆપેલ સંયોજનો માટે સંસ્પંદન ઉર્જાનો યોગ્ય ક્રમ કયો છે
- 9View Solutionસૂર્યપ્રકાશ વડે કેવા પ્રકારનું વિભાજન શક્ય છે ?
- 10View Solutionકયામાં દ્વિ-ધ્રુવીય ચાકમાત્રા મહત્તમ છે?
