$NF_3$ની દ્વિધ્રુવીય ચાકમાત્રા એ કોના કરતાં નાની છે
Medium
a
Both the molecules have a pyramidal shape with a lone pair of electrons on the nitrogen atom. Although fluorine is more electronegative than nitrogen, the resultant dipole moment of \(\mathrm{NH}_{3}\left(4.90 \times 10^{-30} \mathrm{CM}\right)\) is greater than that of \(\mathrm{NF}_{3}\left(0.8 \times 10^{-30} \mathrm{Cm}\right)\)
Both the molecules have a pyramidal shape with a lone pair of electrons on the nitrogen atom. Although fluorine is more electronegative than nitrogen, the resultant dipole moment of \(\mathrm{NH}_{3}\left(4.90 \times 10^{-30} \mathrm{CM}\right)\) is greater than that of \(\mathrm{NF}_{3}\left(0.8 \times 10^{-30} \mathrm{Cm}\right)\)
This is because in case of \(\mathrm{NH}_{3}\) the orbital dipole due to lone pair is in the same direction as the
resultant dipole moment of the \(\mathrm{N}-\mathrm{H}\) bonds. Whereas in \(\mathrm{NF}_{3}\) the orbital dipole is in the direction opposite to the resultant dipole moment of
the three \(N\) - F bonds. The orbital dipole because of the lone pair decreases the effect of the
resultant \(N\) - F bond moments, which results in the low dipole moment of \(\mathrm{NF}_{3}\).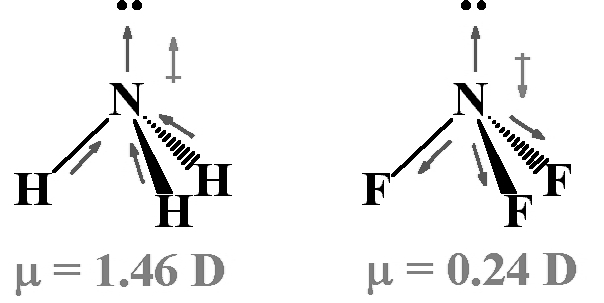
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$s{p^3}$સંકર કક્ષક શું ધરાવે છે?View Solution
- 2$PCl _{5}, BrF _{5}, \& IF _{7}, BrF _{5} \& IF _{7}$ ના આકારો સંબંધિત યોગ્ય વિધાન પસંદ કરો:View Solution
- 3$PCl_5$ માટે નીચેનામાંથી કયું વિધાન ખોટું છે?View Solution
- 4View Solutionનીચેનામાંથી કોણ રેખીય નથી ?
- 5$p - p$ કક્ષકોના બાજુએથી ઓવરલેપ થવાથી નીચેનામાંથી ક્યો બંધ બનશે?View Solution
- 6View Solutionનીચેનામાંથી ક્યો અણુ/આયન અયુગ્મિત ઇલેક્ટ્રોન ધરાવતો નથી ?
- 7View Solutionનીચેનામાથી કયૂ ખોટું વિધાન છે?
- 8View Solutionનીચેના માંથી ક્યાં વધુ સહસંયોજક બંધ વાળા અણુ છે
- 9View Solutionનીચે પૈકી કયામાં મોટો બંધકોણ છે?
- 10View Solutionશૂન્ય દ્વિધ્રુવીય ચાકમાત્રા સાથે ઝેનોન ધરાવતું સંયોજન કયું છે?
