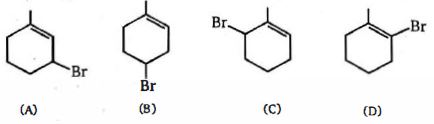
નીચે આપેલી જોડીઓ માં $(A)$ અને $(B)$ માં વધુ એસિડિક અને $(C)$ અને $(D)$ માં વધુ બેઝિક શોધો
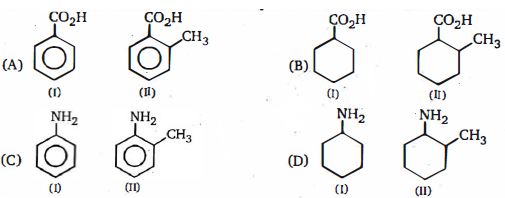

Diffcult
b
\((b)\) The ortho-effect: This is a special effect that is shown by \(o\) -sbstituents of benzene and its derivatives, but is not necessarily just \(a'\) steric effect, e.g, the basicities of some \(o\) -substituted anilines were explained in terms of steric effects and differences in crowding round the nitrogen atom. - This ortho-effect also operates with the benzoic acids. Irrespective of the polar type, nearly all \(o\)-substituted benzoic acids are stronger than benzoic acid. As w.e have seen, benzoic acid is a resonance hybrid, and so the carboxyl groups is coplanar with the ring. An \(o\) -substituent tends to prevent this coplanarity. Thus resonance is diminished (or prevented), and so the \(o\) -atom of the \(OH\) groups has a greater positive charge, resulting increased acid strength. It follows from this that the greater the steric inhibition to resonance, the stronger is the acid. Support for this is the fo llowing order of strengths of substituted benzoic acids.
\((b)\) The ortho-effect: This is a special effect that is shown by \(o\) -sbstituents of benzene and its derivatives, but is not necessarily just \(a'\) steric effect, e.g, the basicities of some \(o\) -substituted anilines were explained in terms of steric effects and differences in crowding round the nitrogen atom. - This ortho-effect also operates with the benzoic acids. Irrespective of the polar type, nearly all \(o\)-substituted benzoic acids are stronger than benzoic acid. As w.e have seen, benzoic acid is a resonance hybrid, and so the carboxyl groups is coplanar with the ring. An \(o\) -substituent tends to prevent this coplanarity. Thus resonance is diminished (or prevented), and so the \(o\) -atom of the \(OH\) groups has a greater positive charge, resulting increased acid strength. It follows from this that the greater the steric inhibition to resonance, the stronger is the acid. Support for this is the fo llowing order of strengths of substituted benzoic acids.
\(2,6 - di - Me (pK_a \,3.21) > 2 - t - Bu \,(pK_a \,3.46) > 2 - Me \,(pK_a\, 3.91)\).
Here again, if we consider the stability of the anion, steric inhibition of resonance prevents the \(+ R\) effect of the ring coming into operation (see above), and since this weakens acid strength, its absence results in increased acid strength.
For options \(B\) and \(D\), no ortho effect is valid and order of acidity and basicity is calculated by nearly examining the inductive effect.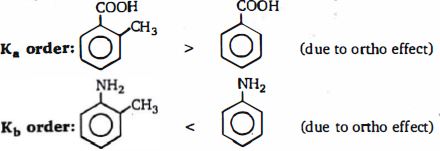
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઆલ્કીન સાથે બ્રોમિનની પ્રક્રિયા એ શેનું ઉદાહરણ છે ?
- 2View Solutionનીચે આપેલા માટે સૌથી વધુ સ્થાયી કાર્બોકેશાયન શોધી.
- 3નીચે આપેલા $H$ પરમાણુઓનો ચઢતો એસિડિકતતા ક્રમ શોધો.View Solution
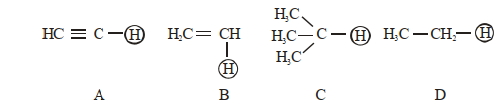
- 4View Solutionનીચેનામાંથી કયો સૌથી વધુ સ્થાયી ધનાયન છે?
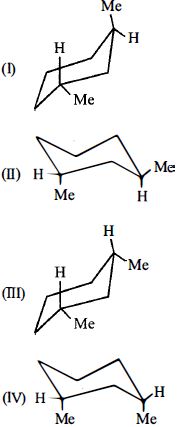
- 5View Solutionનીચેના અણુઓ માટે સ્થાયીતાનો સાચો ક્રમ (ઘટતો ક્રમ) ગોઠવો.
- 6View Solutionનીચેનામાંથી કોણ મહત્તમ કેન્દ્રાનુરાગીતા ધરાવે છે ?
- 7View Solutionનીચેનામાંથી કયો ઇલેક્ટ્રોન અનુરાગી નથી?
- 8View Solutionકેન્દ્ર અનુરાગીનો સાચો ક્રમ કયો છે.
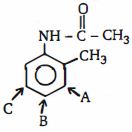
- 9ઇલેક્ટ્રોનઅનુરાગી ચક્રીયવિસ્થાપન $(EAS)$ સૌથી વધુ અનુકૂળ છે તે સ્થાનને ઓળખો.View Solution
- 10View Solutionઆપેલ સંયોજનમાં બે ને પસંદ કરો જે આયનીકરણ પર સમાન કાર્બોકેટાયનઆપે છે.