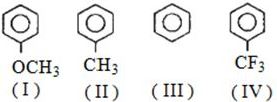
નીચે પૈકી કોનો વિયોજન અચળાંક સૌથી ઉચ્ચ છે?
AIIMS 2004, Diffcult
d
(d) Dissociation of proton from $C{H_3} - \mathop N\limits^ + {H_3}C{l^ - }$ is very difficult due to $-I$ effect of $C{l^ - }$ and ${N^ + }$ while in ${C_6}{H_5}OH$ due to resonance stabilization of phenoxide ion proton eliminate easily similarly due to $H-$ bonding in ${C_6}{H_5}C{H_2}OH$ it can be eliminate and $C{H_3}C \equiv CH$ show acidic character by triple bond by which proton can be dissociate.
(d) Dissociation of proton from $C{H_3} - \mathop N\limits^ + {H_3}C{l^ - }$ is very difficult due to $-I$ effect of $C{l^ - }$ and ${N^ + }$ while in ${C_6}{H_5}OH$ due to resonance stabilization of phenoxide ion proton eliminate easily similarly due to $H-$ bonding in ${C_6}{H_5}C{H_2}OH$ it can be eliminate and $C{H_3}C \equiv CH$ show acidic character by triple bond by which proton can be dissociate.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કયું વિધાન સાચું છે ?
- 2નીચેનામાંથી કયો સમૂહ $-I$ અને $o, p$ નિર્દેંશક તરીકે પણ કાર્ય કરે છે ?View Solution
- 3નીચેનાને તેમના $pK_a$ મૂલ્યોના વધતા ક્રમમાં ગોઠવોView Solution
$(x)\begin{array}{*{20}{c}}
{O\,\,\,}\\
{||\,\,\,}\\
{C{H_3} - S - O - H}\\
{||\,\,\,\,}\\
{O\,\,\,\,}
\end{array}$$\begin{array}{*{20}{c}}
{\,\,\,\,\,O}\\
{\,\,\,\,\,\,||}\\
{(y)\,\,\,C{H_3} - C - O - H}
\end{array}$$(z)\,\, CH_3 -OH$
- 4નીચેની પ્રક્રિયાઓ માટે કયું વિધાન સાચું છે?View Solution
$(A)\,\,CH_3CH_2CH_2Br + KOH \rightarrow CH_3CH CH_2 + KBr + H_2O$
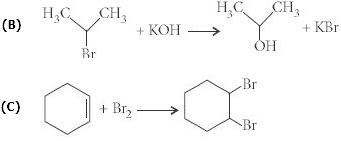
- 5View Solutionનીચેના પૈકી સંયોજનોમાંથી હાઇડ્રોક્સિલ આયનો દ્વારા કેન્દ્રાનુરાગી હુમલો માટે પ્રતિરોધક છે?
- 6કયા સમૂહની જોડી $ (-I) $ અસર દર્શાવે છે ?View Solution
- 7નીચે આપેલી જોડીઓ માં $(A)$ અને $(B)$ માં વધુ એસિડિક અને $(C)$ અને $(D)$ માં વધુ બેઝિક શોધોView Solution
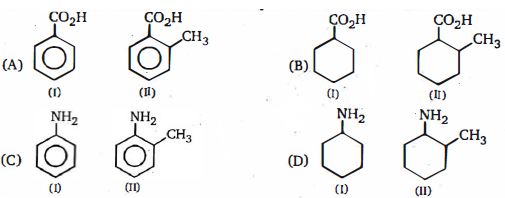
- 8કયો દ્વિતીયક મૂલક છે ?View Solution
$(1)$ $C{H_3} - \mathop {CH}\limits^| - {C_2}{H_5}$
$(2)$ $C{H_2} = \,\mathop C\limits^| \, - \,\,C{H_3}$
$(3)$ $ CH_2 = CH -$
$(4)$ $(CH_3)_2 CH -$
- 9View Solutionઆપેલા પ્રમાણભૂત બંધારણણો સાચો સ્થાયિતા ક્રમ કયો છે ?
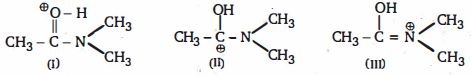
- 10View Solutionનીચેના પદાર્થોમાં કેન્દ્રાનુરાગી વિસ્થાપન માટે પ્રક્રિયાશીલતાનો ઘટતો ક્રમ કયો છે ?