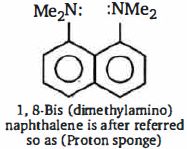
તેની મૂળ પ્રબળતા $I$ - ડાયમિથાઇલ એમિનો નેપ્થાલિન કરતા વધુ $10^{10}$ છે. ઉચ્ચ બેઇઝ પ્રબળતા નું કારણ છે

Diffcult
b
\((b)\) Steric effects can indirectly affect acidity or basicity by affecting the resonance. Steric effects can also be caused by other types of strain. \(1,8\) - bis(diethylamino)- \(2, 7\) -dimethoxynaphthalene \((1) \) is an extremely strong base for a tertiary amine (\(pK_a\) of the conjugate acid \(= 16.3\); compare \(N, N\) - dimethylaniline , \(pK_a = 5.1\)), but proton transfers to and from the nitrogen are exceptionaly slow ; enough to be followed by a \(uv\) -spectrophotometer. \(1\) is severely strained because the two nitrogen lorie pairs are forced to be near each other. Protonation relieves the strain : one lone pair is now connected to a hydrogen, which forms a hydrogen bond to the other lone pair (shown in \(2\)). The same effects are found in \(4, 5\) -bis (dimethylamino) fluorene \((3)\) and \(4\), \(5\) - bis (dimethylamino).
\((b)\) Steric effects can indirectly affect acidity or basicity by affecting the resonance. Steric effects can also be caused by other types of strain. \(1,8\) - bis(diethylamino)- \(2, 7\) -dimethoxynaphthalene \((1) \) is an extremely strong base for a tertiary amine (\(pK_a\) of the conjugate acid \(= 16.3\); compare \(N, N\) - dimethylaniline , \(pK_a = 5.1\)), but proton transfers to and from the nitrogen are exceptionaly slow ; enough to be followed by a \(uv\) -spectrophotometer. \(1\) is severely strained because the two nitrogen lorie pairs are forced to be near each other. Protonation relieves the strain : one lone pair is now connected to a hydrogen, which forms a hydrogen bond to the other lone pair (shown in \(2\)). The same effects are found in \(4, 5\) -bis (dimethylamino) fluorene \((3)\) and \(4\), \(5\) - bis (dimethylamino).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેનામાંથી કયો સેન્ડ મેયર પ્રકિયા માં મધ્યવર્તી શું હશે ?View Solution
$(i)$ ${C_6}{H_5}{N^ + } \equiv NC{l^ - }$ $(ii)$ ${C_6}{H_5}{N^ + } \equiv N$
$(iii)$ ${{\overset{\centerdot }{\mathop{C}}\,}_{6}}{{H}_{5}}$ $(iv)$ $C_6H_5Cl$ - 2View Solutionકયો કાર્બોનીયમ આયન સૌથી ઓછો સ્થિર છે ?
- 3વધતા દરના ક્રમમાં નીચેની પ્રતિક્રિયાઓ $A, B$ અને $C$ ને યોગ્ય ક્રમ આપો,View Solution
- 4View Solutionઆપેલ ધનાયનમાં, સૌથી સ્થાયી કાર્બોનિયમ આયન કયો છે?
- 5નીચેના માટે એસિડિક ક્ષમતાનો સાચો ક્રમ લખોView Solution
$(I)$ $ CH_3 - NO_2$
$(II)$ $NO_2 - CH_2 - NO_2$
$(III)$ $ CH_3 - CH_2 - NO_2 $
$(IV)$ $\begin{array}{*{20}{c}}
{N{O_2} - CH - N{O_2}} \\
{|\,\,\,\,\,\,} \\
{\,N{O_2}}
\end{array}$ - 6$A, B,$ and $C$ની વચ્ચે કાર્બન-કાર્બન બંધ પરિભ્રમણની તુલના કરોView Solution
- 7કાર્બેનાયન્સની સ્થાયિતાનો ક્રમ શું છે?View Solution
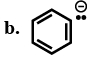
$(i)\,\,RC \equiv \mathop C\limits^ \ominus $
$(ii)\,\,[IMAGE]$
$(iii)\,\,{R_2}C = \mathop C\limits^ \ominus H$
$(iv)\,\,{R_3}C - \mathop C\limits^ \ominus {H_2}$
- 8View Solutionનીચે પૈકી કોની દ્વિધ્રુવીય ચાકમાત્રા સૌથી વધારે છે?
- 9$1-$મિથોક્સી$-1, 3-$બ્યુટાડાઇનના નીચેનામાંથી સંસ્પંદનીય રચનાઓમાંથી કયું ઓછામાં ઓછું સ્થાયી છે?View Solution
- 10ઈલેકેટ્રોન અનુરાગી $ E^{\oplus} $ બેન્ઝિન ચક્ર પર હુમલો કરીને મધ્યવર્તીં $ \sigma -$ સંકીર્ણ બનાવે છે. તેમાંથી કયો $\sigma - $ સંકીર્ણ સૌથી ઓછી ઉર્જા ધરાવે છે ?View Solution
