નીચેના બ્રોમાઈડને ધ્યાનમાં લો.
$S_N1$ પ્રતિક્રિયાનો સાચો ક્રમ કયો છે?
AIIMS 2016, Medium
a
Since \(S_N1\) reactions involve the formation of carbocation as intermediate in the rate determining higher will be the reactivity of alkyl halides toward \(S_N1\) route. Now we know that stability of carbocations follows the order \(:\,3^o>2^o>1^o,\) so \(S_N1\) reactivity should also follow the same order.
Since \(S_N1\) reactions involve the formation of carbocation as intermediate in the rate determining higher will be the reactivity of alkyl halides toward \(S_N1\) route. Now we know that stability of carbocations follows the order \(:\,3^o>2^o>1^o,\) so \(S_N1\) reactivity should also follow the same order.
\(3^o > 2^o > 1^o >\) Methyl (\(S_N1\) reactivity)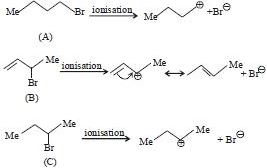
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$n$ પ્રોપાઇલ બ્રોમાઇડની આલ્કોહોલિક પોટેશિયમ હાઇડ્રોક્સાઇડ સાથેની પ્રક્રિયાથી ........... બને છે.View Solution
- 2View Solutionસેન્ડમેયર પ્રક્રિયા વડે બનતા નીચે આપેલામાંથી હેલોબેન્ઝિન ની સંખ્યા ............. છે. (નજીકનું પૂર્ણાક)

- 3$\,$View Solution

- 4સિલ્વર એસીટેટ + $B{{r}_{2}}\xrightarrow{\text{C}{{\text{S}}_{\text{2}}}}\,\,\,......$ આ પ્રક્રિયા ની મુખ્ય નીપજછે.View Solution
- 5$1, 3-$ ડાયબ્રોમો પ્રોપેનની ધાત્વીય ઝિંક સાથેની પ્રક્રિયા .......... આપે છે.View Solution
- 6$C{H_3}C{H_2}CH(F)C{H_3}$ ની $C{H_3}{O^ - }/C{H_3}OH$ સાથેની પ્રક્રિયાથી મુખ્ય નીપજ .......... મળશે.View Solution
- 7View Solutionફ્રીઓન (ડાયક્લોરોડાયફ્લોરો મિથેન) નો ઉપયોગ શું થાય છે?
- 8સમધટકીય મોનોક્લોરો વ્યુતપન્નોની મહત્તમ સંખ્યા કે જે ને $2,2,5,5-$ ટેટ્રામિથાલહેકઝેનના કલોરિનેશન વડે મેળવી શકાય છે $..............$ છે.View Solution
- 9$4-t$ -બ્યુટાઇલસાયકલોહેકઝાઇલ આયોડાઇડ $(^{127}I^-)$ ના બે અવકાશીય સમઘટકમાંથી કયું $S_{N^2}$ , $^{128}I^-$ સાથે વિસ્થાપન કરશે,View Solution

- 10View Solutionનીચેનામાંથી ક્યો કાર્બોકેટાયન ફરીથી ગોઠવણ કરશે ?
