$(i)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}-CH_2} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}}
\end{array}$
$(ii)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{H}}
\end{array}$
$(iii)$ $\begin{array}{*{20}{c}}
{\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,|}
\end{array}} \\
{C{H_3} - {C^ + }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}}
\end{array}$
$(iv)$ $Ph - CH ^{+}-\underline{ CH }_{3}$
The stability of carbocation depends upon $+ M$ effect, $+ I$ effect and $+ H$ effect.
Carbocation $(i)$ shows eight hyperconjugation structures.
Carbocation $(ii)$ shows six hyperconjugation structures.
Carbocation $(iii)$ shows nine hyperconjugation structures.
Carbocation $(iv)$ shows $+M$ effect.
Therefore, the correct stability order is : $iv > iii > i > ii$
Download our appand get started for free
Similar Questions
- 1View Solutionઆપેલ સંયોજન માટે વાસ્તવિક બંધારણ અને નીચી ઉર્જાવાળા સસ્પંદન બંધારણ વચ્ચે ઊર્જામાં તફાવત છે તે_________
- 2View Solutionતીર દ્વારા સૂચવવામાં આવેલ હાઇડ્રોજન ક્યાથી સરળતાથી દૂર થઈ જશે
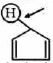
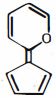
- 3View Solutionઇલેકટ્રોનઅનુરાગી ચક્રીય વિસ્થાપન માં દરનો ઘટતો ક્રમ કયો છે ?

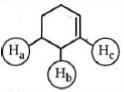
- 4એસિડિક પ્રબળતા અનુસાર નીચેના પરમાણુઓમાં હાઇડ્રોજન અણુઓ $(H_a , H_b, H_c,)$ ને ક્રમ આપોView Solution
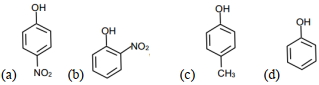
- 5View Solutionએસિડિક સ્વભાવનો ક્રમ શું હશે ?
- 6નીચેના કેન્દ્રાનુરાગીમાંથી કેન્દ્રાનુરાગીનો ઘટતો ક્રમ કયો છે ?View Solution
$1.\,\,\,\begin{array}{*{20}{c}}
{C{H_3} - C - {O^ - }} \\
{\,\,\,||} \\
{\,\,\,O}
\end{array}$ $2.\,\,CH_3O^-$ $3.\,\,CN^-$
- 7View Solutionનીચેના પૈકી કયો કાર્બોકેટાયન સૌથી વધુ સ્થાયી છે?
- 8View Solutionસૌથી પહેલા હાઇડ્રોજનની ઉષ્મામાં ઘટાડો કરવાના ક્રમમાં નીચે આપેલા આલકેન્સને ક્રમ આપો
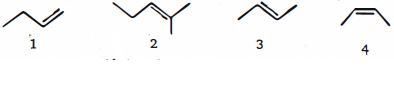
- 9View Solutionનીચેનામાંથી કયા સંયોજન પર ઇલેક્ટ્રોન અનુરાગી પ્રક્રિયક દ્વારા સરળતાથી હુમલો કરવામાં આવશે?
- 10View Solutionઆપેલ અણુની સૌથી સ્થાયી પ્રમાણભૂત રચના કઈ છે: