નીચેના પૈકી ક્યું સમચતુષ્ફલકીય આકાર ધરાવતું નથી ?
JEE MAIN 2014, Diffcult
b
In case of \(N(SiH_3)_3\, N\) atom is \(sp^2\) hybridised, the lone pair is present in \(2p\) orbital and it is transferred to empty \(d\) orbital of \(Si\) forming \(d\pi -p\pi \) bond . Hence nitrogen with \(sp^2\) hybridization has trigonal planar shape.
In case of \(N(SiH_3)_3\, N\) atom is \(sp^2\) hybridised, the lone pair is present in \(2p\) orbital and it is transferred to empty \(d\) orbital of \(Si\) forming \(d\pi -p\pi \) bond . Hence nitrogen with \(sp^2\) hybridization has trigonal planar shape.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી ક્યા પ્રકારની કક્ષકોના ઓવરલેપીંગથી પાઇ બંધ બને છે?
- 2નીચે આપેલામાંથી વળેલ-આકારના અણુ(ઓ)ની સંખ્યા $.......$ છે.$N _3^{-}, NO _2^{-}, I _3^{-}, O _3, SO _2$View Solution
- 3View Solutionનીચેનામાંથી કોણ રેખીય નથી ?
- 4${H_2}{O_2}$ માં બે ઓક્સિજન પરમાણુઓ વચ્ચે શું હોય છેView Solution
- 5View Solutionનીચેના પૈકી કયુ આણ્વિય કક્ષકની આકૃતિને સૌથી સારી રીતે રજૂ કરે છે ?
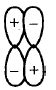
- 6View Solutionનીચેના સંયોજન પૈકી આયનીય,સહ-સંયોજક ,સવર્ગ સહ-સંયોજક તેમજ હાઈડ્રોજનબંધ ધરાવેછે?
- 7${H_2}{O_2}$ માં બે ઓક્સિજન પરમાણુઓ વચ્ચે શું હોય છેView Solution
- 8$\mathrm{NH}_{3}, \mathrm{H}_{2},\mathrm{O}_{2}$ અને $\mathrm{CO}_{2}$ ના વાન ડર વાલ્સ અચળાંક અનુક્રમે $4.17,0.244,1.36$ અને $3.59$ આપેલ છે. નીચે આપેલા વાયુઓ પૈકી ક્યા એકનું પ્રવાહીકરણ સૌથી સરળતાથી થશે ?View Solution
- 9$HF$ માં બંધઅંતર $9.17\times10^{-11}\, m$ છે. $HF$ ની દ્વિધ્રુવ ચાકમાત્રા $6.104\times10^{- 30}\, Cm$ છે, તો $HF$ માં ટકાવાર આયનીય લાક્ષણિકતા જણાવો. (ઇલેક્ટ્રોન વીજભાર $= 1.60\times10^{-19}\, C$)View Solution
- 10પાણી $\left( {{H_2}O} \right)$ એ પ્રવાહી છે જ્યારે હાઇડ્રોજન સલ્ફાઇડ $\left( {{H_2}S} \right)$ વાયુ છે તેનું કારણ ......View Solution
