નીચેના પૈકી સૌથી ઓછી દ્વિધ્રુવ ચાકમાત્રા ધરાવતો અણુ જણાવો.
AIEEE 2012, Medium
d
\(CCl_4\) has lowest (zero) dipole moment. This is due to its symmetrical tetrahedral structure. Due to which dipole moment of one bond is cancelled by opposite dipole moment of the other three bonds..
\(CCl_4\) has lowest (zero) dipole moment. This is due to its symmetrical tetrahedral structure. Due to which dipole moment of one bond is cancelled by opposite dipole moment of the other three bonds..
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$HCOO^-$ એનાયનમાં બે કાર્બન - ઓક્સિજન બંધોએ સમાન લંબાઈમાં જોવા મળે છે તો તેનું કારણ શું હશે ?View Solution
- 2કયા સમૂહની જોડી $ (-I) $ અસર દર્શાવે છે ?View Solution
- 3નીચેનામાંથી કયા પરમાણુમાં શૂન્ય સિવાયના દ્વિધ્રુવી ચાકમાત્રા છે?View Solution
$(I)$ $1, 2$ - ડાયબ્રોમોઇથેન ની ગૌચ રચના
$(II)$ $1, 2$ -ડાયબ્રોમોઇથેન ની એન્ટિ રચના
$(III)$ ટ્રાન્સ - $1, 4$ -ડાયબ્રોમોસાયકલોહેકઝેન
$(IV)$ સિસ - $1, 4$ -ડાયબ્રોમોસાયકલોહેકઝેન
$(V)$ ટેટ્રાબ્રોમોઇથેન
$(VI)$ $1, 1$ - ડાયબ્રોમોસાયકલોહેકઝેન
- 4$p$ નાઈટ્રોફિનોકસાઈડ આયનનું સંસ્પદન બંધારણનું સૌથી યોગ્ય નિરૂપણ કયું છે ?View Solution
- 5આપેલ સંયોજનો માટે, ચઢતા $\mathrm{pK}_{\mathrm{a}}$ મૂલ્ય નો સાચો ક્રમ શોધો ?View Solution
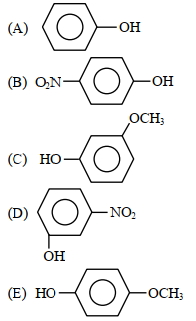
- 6View Solutionઇલેકટ્રોનઅનુરાગી ચક્રીય વિસ્થાપન માં દરનો ઘટતો ક્રમ કયો છે ?

- 7$Cl^-, CH_3COOC^-,OH^- $ અને $ F^- $ ની બેઝિક ક્ષમતાનો વધતો ક્રમ કયો છે ?View Solution
- 8View Solutionકેન્દ્ર અનુરાગીનો સાચો ક્રમ કયો છે.
- 9View Solutionસ્થાયી કાર્બોકેટાયન જે ઉપરની પ્રક્રિયામાં બને છે તે શોધો.
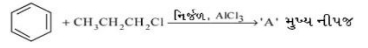
- 10બેઇઝની પ્રબળતાનો ક્રમ કયો છે?View Solution
$\mathop {{H_3}C\mathop C\limits^ \ominus {H_2}}\limits_{(i)} \,,$$\mathop {{H_2}C = \mathop C\limits^ \ominus H}\limits_{(ii)} $ અને $\mathop {H - C \equiv \mathop C\limits^ \ominus }\limits_{(iii)} $
