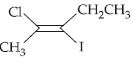
નીચેના સંયોજનનું $IUPAC$ નામ શું છે?

AIPMT 1998,AIPMT 2011, Medium
a
The parent hydrocarbon contains \(5 C\) atom continuous chain with one \(C = C\) bond between second and third carbon atom. Hence, it is named as \(2\)-pentene.
The parent hydrocarbon contains \(5 C\) atom continuous chain with one \(C = C\) bond between second and third carbon atom. Hence, it is named as \(2\)-pentene.
One \(Cl\) and one \(I\) are present at second and third carbon atom respectively. The molecule has trans geometry across \(C = C\) bond.
Hence, it is named trans-\(2\)-chloro-\(3\)-iodo-\(2\)-pentene.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1પદાર્થોનું $IUPAC$ નામ લખો.View Solution
- 2ટ્રાન્સ$-2-$હેકઝેનાલનું સાચું બંધારણ કયું છે ?View Solution
- 3પદાર્થનું $IUPAC$ નામ જણાવો.View Solution
- 4નીયો પેન્ટેનનું $IUPAC$ નામ જણાવો.View Solution
- 5આપેલ પદાર્થનું $ IUPAC$ નામ શું છે ?View Solution
- 6View Solutionઇથાઈન ના સંદર્ભમાં ખોટું વિધાન શોધો.
- 7પદાર્થનું સાચું $IUPAC$ નામ જણાવો.View Solution
$\begin{array}{*{20}{c}}
{Me} \\
{|\,\,\,\,} \\
{{H_2}C - N - C - Et} \\
{\,\,\,\,\,\,|\,\,\,\,\,\,\,\,\,|\,\,} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,Me\,\,\,\,\,C{H_2}C{H_3}}
\end{array}$ - 8નીચેના પદાર્થનું $IUPAC$ નામ કયું છે ?View Solution
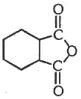
- 9નીચેના સંયોજનનું સાચું નામ $IUPAC$ જણાવો.View Solution
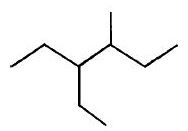
- 10નીયોપેન્ટેનનું $IUPAC$ નામ જણાવો.View Solution
