$(I)$ $C{H_2} = CH\mathop C\limits^ + HC{H_3}$
$\begin{array}{*{20}{c}}
{{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,} \\
{(II)\,\,\,\,\,\,\,\,\,\,C{H_2} = C - \mathop {{\text{ }}C}\limits^ + {H_2}}
\end{array}$
$(III)$ $C{H_3}CH = CH\mathop C\limits^ + {H_2}$
Let us first write the resonance hybrid of the three allyl carboniun ions.
$\underbrace{\stackrel{1}{C} H_{2}=\;\stackrel{...2}CH-\stackrel{...3}C}_{\stackrel{\oplus}I} H \leftarrow C H_{3}$
$C H_{3} \rightarrow \underbrace{\stackrel{3}C H-\stackrel{2}C H-\stackrel{1}C H_{2}}_{\stackrel{\oplus}{I I I}}$
We know that better the dispersal of $+$ charge, more will be the stability of the carbonium ion. Further, we know that $C_1$ and $C_3$ carry most of the positive charge which is
$\stackrel{1}{C} H_{2}=\stackrel{2}{C} H-{\stackrel{3}{C}} \;^\oplus H \leftarrow C H_{3} \leftrightarrow$ $\stackrel{1}{C}\,^\oplus H_{2}-\stackrel{2}C H=\stackrel{3}C H-C H_{3}$
dispersed by the methyl group ( $+ I$ group) present on $I$ and $II$, thus these two are more and equally stable than the $II$ in which methyl group is present on $C_2$ which carry little of the positive charge.
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કયો ઇલેક્ટ્રોન અનુરાગી નથી?
- 2View Solutionવધુ એસિડિક કયું છે ?
- 3View Solutionભાગ લેતા બંધારણોની સાપેક્ષ સ્થિરતા શોધો :
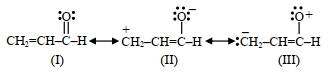
- 4$2-$બ્રોમો પેન્ટનની ડિહાઈડ્રોહેલોજીનેશન પ્રક્રિયામાં મુખ્ય નીપજ પેન્ટ$-2-$ઈન બને છે. આ બનતી નિપજ આધારિત છે તે $....\,.$View Solution
- 5$(I)$ $CH_3CH_2COOH $View Solution
$(II)$ $CH_2 = CH - COOH $ અને
$(III)$ $ HC \equiv C - COOH$
એસિડોની ગોઠવણી ઘટતી એસિડીકતા ક્રમમાં ગોઠવો.
- 6View Solutionનીચેનામાંથી કયો સૌથી સ્થિર કાર્બોએનાયન છે ?
- 7આપેલ સંયુગ્મિત બેઈઝ $ (R = CH_3) $ ની વધતી બેઝીકતાનો સાચો ક્રમ કયો છે ?View Solution
- 8View Solutionહાઇડ્રેટની રચના માટે સંતુલન સતતનો વધતો ક્રમ કયો છે
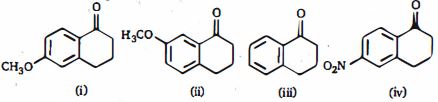
- 9View Solutionનીચેનામાંથી કયો કેન્દ્રાનુરાગી દ્વારા સૌથી સરળ રીતે હુમલો કરી શકે છે ?
- 10View Solutionનીચેના પદાર્થો માટે એસિડિક ક્ષમતાનો ક્રમ કયો છે ?