નીચેનામાંથી કઈ પ્રક્રિયા એ કેન્દ્રાનુરાગી વિસ્થાપન પ્રક્રિયાનું ઉદાહરણ છે?
AIPMT 2009, Diffcult
d
$K O H \rightarrow K^{+}+O H^{-}$
$K O H \rightarrow K^{+}+O H^{-}$
$RX + \mathop {O{H^ - }}\limits_{Nucleophile} \to R - OH + {X^ - }$
$O^{-}$ stronger nucleophile than halogen so, easily replace the weaker nucleophile. Nucleophiles are either negative charge or lone pair of electrons bearing species, e.g. $O H^{-} ; \ddot{N H}_{3},$ etc
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$- M$ અને ઋણ પ્રેરક અસરોના અસ્તિત્વ માટે વિસ્થાપીત સમૂહોનો યોગ્ય સેટ પસંદ કરો.View Solution
- 2ટોલ્યુઈનમાં $ (+) - 1 - $ક્લોરો $-1 - $ ફિનાઈલ ઈથેનનું દ્રાવણનું $ SbCl_5 $ ના થોડા મૂૂલ્યની હાજરીમાં ધાતુ રેસેમિકરણ થાય છે કારણ કે તેમાં......નું નિર્માણ થાય છે ?View Solution
- 3View Solutionનીચેનામાંથી કયો સૌથી એસિડીક પદાર્થ છે ?
- 4$D\,(+)$ $- 2-$ક્લોરો$-2-$ફિનાઇલ ઇથેનનું ટોલ્યુઇનમાં દ્રાવણ, $SbC{l_5}$ની ઓછી માત્રાની હાજરીમાં શું બનાવીને રેસેમીકૃત બને છે?View Solution
- 5$\mathop {C{H_2} = CH - CH = CH{ - ^ \oplus }N{H_3};}\limits_{(I)} \,\,\mathop {{\,^ \oplus }C{H_2}-CH = CH{ - ^\circleddash }CH{ - ^ \oplus }N{H_3}}\limits_{(II)} $View Solution
$\mathop {^ \oplus C{H_2} - CH = CH - CH = N{H_3}}\limits_{(III)} $
આમાંથી કયું બંધારણ માન્ય માન્ય પ્રામાણભૂત બંધારણ નથી?
- 6બેઇઝની પ્રબળતાનો ક્રમ કયો છે?View Solution
$\mathop {{H_3}C\mathop C\limits^ \ominus {H_2}}\limits_{(i)} \,,$$\mathop {{H_2}C = \mathop C\limits^ \ominus H}\limits_{(ii)} $ અને $\mathop {H - C \equiv \mathop C\limits^ \ominus }\limits_{(iii)} $
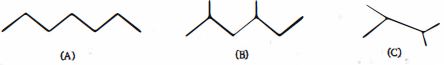
- 7દહન ઉષ્માના ક્રમમાં નીચેના પદાર્થોને ક્રમાંકિત કરો ( મોટાભાગના ઉષ્માશોષક $\to$ ઓછામાં ઓછા ઉષ્માશોષક)View Solution
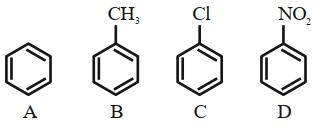
- 8$Br_2/ AlCl_3$ ની સાથે પ્રક્રિયા દરનો વધતો ક્રમ છેView Solution
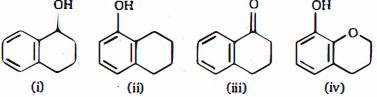
- 9View Solutionનીચેનામાંથી સંયોજન માંથી કયા સંયોજન સૌથી ઝડપી દરે તેની ચક્રીય રીંગ (ઇલેક્ટ્રોઅનુરાગી ચક્રીય વિસ્થાપન ) ની બ્રોમિનેશનમાંથી પસાર થાય છે?
- 10View Solutionઈલેકટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયા પ્રત્યેની સક્રિયતાનો ક્રમ જણાવો.