નીચેનામાંથી કઈ રાસાયણિક પદ્ધતિ અ-ચક્રીય છે?
NEET 2013, Medium
d
The molecules which do not satisfy Huckel rule or $(4 n+2) \pi$ -electron rule are said to be nonaromatic. The compound (d) has total $4 \pi e^-$. It does not follow $(4n + 2)$ rule. So it is non-aromatic compound. All other compounds $(a, b, c)$ are planar and have $6 \pi e^{-},$ so they are aromatic.
The molecules which do not satisfy Huckel rule or $(4 n+2) \pi$ -electron rule are said to be nonaromatic. The compound (d) has total $4 \pi e^-$. It does not follow $(4n + 2)$ rule. So it is non-aromatic compound. All other compounds $(a, b, c)$ are planar and have $6 \pi e^{-},$ so they are aromatic.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$p$-નાઇટ્રોફીનોકસાઇડ આયનના સસ્પંદન બંધારણના સૌથી અસમાન નિરુપણ છે ?View Solution
- 2નીચેમાંથી કયો $M.K.$ નિયમને અનુસરતો નથી?View Solution
- 3View Solutionબંધની પ્રબળતાનો સાચો ક્રમ કયો છે ?
- 4હેલોહાઈડ્રીન રચવા માટે સાયક્લોહેક્સિનમાં પાણીમાં $Cl_2$ ના ઇલેક્ટ્રોઅનુરાગી ઉમેરામાં મધ્યવર્તીનું સૌથી સ્થિર સ્વરૂપ કયું છે ?View Solution
- 5View Solutionનીચેની પ્રક્રિયાની મુખ્ય નીપજ શું છે?
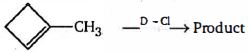
- 6નીચેનામાંથી $(Z)$ નીપજ કઈ હશેView Solution
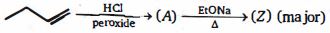
- 7પ્રોપિનના દ્વિબંધ સાથે $HI$ ની યોગશીલ પ્રક્રિયા મુખ્ય નીપજ આઇસો-પ્રોપાઇલ આયોડાઇડ આપે છે. કારણ કે યોગશીલ પ્રક્રિયા ............... દ્વારા થાય છે.View Solution
- 8કઈ પ્રક્રિયા દ્વારા $2$-હેકઝાઈન એ ટ્રાન્સ-$2$-હેકઝીનમાં રૂપાંતર થાય છે ?View Solution
- 9પ્રોપિનના દ્વિબંધ સાથે $HI$ ની યોગશીલ પ્રક્રિયા મુખ્ય નીપજ આઇસો-પ્રોપાઇલ આયોડાઇડ આપે છે. કારણ કે યોગશીલ પ્રક્રિયા ............... દ્વારા થાય છે.View Solution
- 10View Solutionશું થાય છે જ્યારે એસિટિલિન અને હાઇડ્રોજનનું મિશ્રણને ગરમ લિન્ડલરના ઉદીપક પરથી પસાર કરવામાં આવે છે.
