નીચેનામાંથી ક્યા સંયોજનમાંના $C-Cl$ બંધનું આયનીકરણ સૌથી સ્થાયી કાર્બોનિયમ આપશે ?
NEET 2015, Diffcult
d
The stability of carbocation follow the order $3>2>1>$ methyl. More the number of alkyl group attached with the carbon atom carrying the positive charge greater would be the tendency to stabilise positive charge via inductive effect, and hence more stable. Due to the presence of benzence group, there is more resonance possibility than others hence it forms a more stable compound.
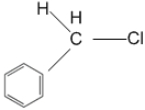
The stability of carbocation follow the order $3>2>1>$ methyl. More the number of alkyl group attached with the carbon atom carrying the positive charge greater would be the tendency to stabilise positive charge via inductive effect, and hence more stable. Due to the presence of benzence group, there is more resonance possibility than others hence it forms a more stable compound.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના પદાર્થો માટે ઈલેકટ્રો અનુરાગી વિસ્થાપન પ્રક્રિયા માટે પ્રક્રિયા શીલતાનો સાચો ક્રમ કયો છે ?
- 2નીચે આપેલ પૈકી કયું સૌથી વધુ સ્થાયી છે $?$View Solution
- 3View Solutionનીચેનીમાંથી કયા ઘટકો ઇલેક્ટ્રોન અનુરાગી સ્વભાવમાં નથી?
- 4View Solutionનીચેની કયો સ્પીસીઝ સૌથી સ્થાયી છે?
- 5View Solutionનીચેના સંયોજનોમાં પ્રોટોનેશનની પ્રાધાન્યવાળી બાજુ કઈ છે
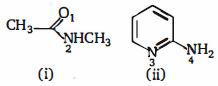
- 6View Solutionનીચેના પૈકી ક્યો સમૂહ બેન્ઝિન વલયને ઇલેક્ટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયા પ્રત્યે નિષ્ક્રિય બનાવે છે ?
- 7નીચે આપેલામાંથી કોના કારણે તૃતીયક બ્યુટાઇલ કાર્બોકેટાયન એ દ્વિતીયક બ્યુટાઇલ કાર્બોકેટાયન કરતા વધુ સ્થાયી છે $?$View Solution
- 8View Solutionઆપેલ સંયોજનોની બેઝિક પ્રબળતાની તુલના કરો:
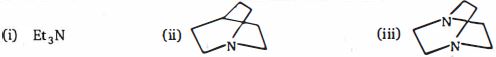
- 9એસિડિક પ્રબળતા અનુસાર નીચેના પરમાણુઓમાં હાઇડ્રોજન અણુઓ $(H_a , H_b, H_c,)$ ને ક્રમ આપોView Solution
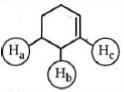
- 10View Solutionનીચેનામાંથી કયા પરમાણુઓમાંના સૌથી મોટા સંસ્પંદન સ્થિર થવાની અપેક્ષા છે
