\((b)\) \((1)\) Nitro-group is electron withdrawing therefore decreases stability.
\((2)\) Methoxy group is electron releasing.
Therefore increases stability by donating electron.
\((3)\) Chlorine is also electron withdrawing but its effect is less than \(-NO_2\) group.
Hence, correct order of stability.
\((4)\)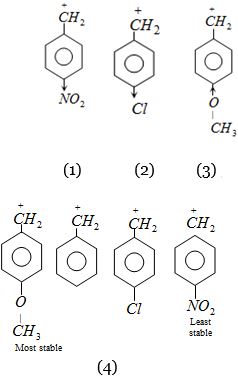
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કઇ સંસ્પંદન રચનાઓ દ્વારા રજૂ કરી શકાતું નથી?
- 2નીચે આપેલ પૈકી, મેટા નિર્દેશક ક્રિયાશીલ સમૂહોની કુલ સંખ્યા __________છે. (પૂણાંક આધારિત)View Solution
$ -\mathrm{OCH}_3,-\mathrm{NO}_2,-\mathrm{CN},-\mathrm{CH}_3-\mathrm{NHCOCH}_3,$ $ -\mathrm{COR},-\mathrm{OH},-\mathrm{COOH},-\mathrm{Cl}$
- 3નીચે આપેલામાંથી કોના કારણે તૃતીયક બ્યુટાઇલ કાર્બોકેટાયન એ દ્વિતીયક બ્યુટાઇલ કાર્બોકેટાયન કરતા વધુ સ્થાયી છે $?$View Solution
- 4આજુબાજુના કેન્દ્રાનુરાગી ની સાચી જોડીઑ કઈ છેView Solution
$(A)$ $AgCN / KCN$
$(B)$ $RCOOAg / RCOOK$
$(C)$ $AgNO _{2} / KNO _{2}$
$(D)$ $AgI / KI$
- 5View Solutionકયો સમૂહ મહત્તમ અતિસંયુગ્મન અસર ધરાવે છે ?
- 6View Solutionનીચેનામાંથી કયા સંયોજન માં સંસ્પંદન સ્થાયી નથી?
- 7$\mathop {C{H_2}}\limits_{(I)} = O \leftrightarrow \mathop {{}^ \oplus C{H_2}}\limits_{(II)} - {O^\circleddash } \leftrightarrow \mathop {{}^\circleddash C{H_2}}\limits_{(III)} - {O^ \oplus }$View Solution
આમાંથી કયું બંધારણ વ્યવહારીક ફોર્માલ્ડિહાઈડ માટે માન્ય પ્રમાણભૂત બંધારણ નથી?
- 8$(I) $ $HCOOH$View Solution
$(II)$ $CH_3COOH$
$(III)$ $ CH_3CH_2COOH $
$(IV)$ $C_6H_5COOH $
પદાર્થો માટે ઘટતી એસિડિકતાનો સાચો ક્રમ કયો છે ?
- 9નીચેનાને તેમના $pK_a$ મૂલ્યોના વધતા ક્રમમાં ગોઠવોView Solution
$(x)\begin{array}{*{20}{c}}
{O\,\,\,}\\
{||\,\,\,}\\
{C{H_3} - S - O - H}\\
{||\,\,\,\,}\\
{O\,\,\,\,}
\end{array}$$\begin{array}{*{20}{c}}
{\,\,\,\,\,O}\\
{\,\,\,\,\,\,||}\\
{(y)\,\,\,C{H_3} - C - O - H}
\end{array}$$(z)\,\, CH_3 -OH$
- 10View Solutionઆ સંયોજનોના હાઇડ્રોજનની ઉષ્મા માટે નીચેનામાંથી કયા ક્રમ યોગ્ય છે?