નીચેનામાંથી ક્યો અણુ મહત્તમ દ્વિધ્રુવ ચાકમાત્રા ધરાવે છે ?
NEET 2014, Medium
c
\(\mathrm{CO}_{2}\) and \(\mathrm{CH}_{4}\) have zero dipole moment as these are symmetrical in nature.
\(\mathrm{CO}_{2}\) and \(\mathrm{CH}_{4}\) have zero dipole moment as these are symmetrical in nature.
Between \(N H_{3}\) and \(N F_{3}, N F_{3}\) has greater dipole mough in \(N H_{3}\) and \(N F_{3}\) both, \(N\) possesses one lone pair of electrons.
This is because in case of \(N H_{3},\) the net \(N\) - \(H\) bond dipole is in the same direction as the direction of dipole of lone pair but in case of \(N F_{3},\) the direction of net bond dipole of three- \(N-F\) bonds is opposite than that of the dipole of the then lone pair.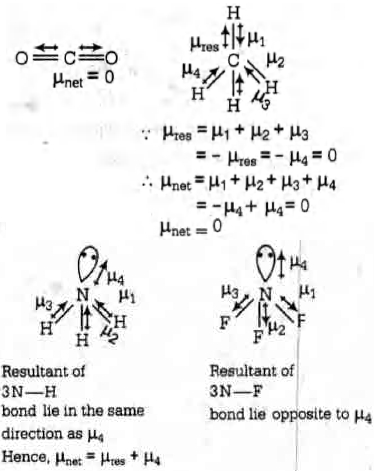
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના ઘટકોમાંથી પ્રતિચુંબકીય અણુ ક્યો છે ?
- 2નીચેનામાંથી કોણ $sp^3$ સંકરણ ધરાવે છે ?View Solution
- 3નીચે આપેલામાંથી અણુઓ અથવા આયનોની સંખ્યા કે જે ઈલેકટ્રોનોની એકી સંખ્યા ધરાવતા નથી તે $.........$View Solution
$(A)$ $NO _2$ $(B)$ $ICl _4^{-}$ $(C)$ $BrF _3$ $(D)$ $ClO _2$ $(E)$ $NO _2^{+}$ $(F)$ $NO$
- 4View Solutionનીચેના માંથી ક્યાં વધુ સહસંયોજક બંધ વાળા અણુ છે
- 5View Solutionઉત્કલનબિંદુનો સાચો ક્રમ મેળવો.
- 6View Solutionનીચેનામાથી ક્યુ વિધાન સત્ય છે ?
- 7View Solutionનીચેના માંથી ક્યાં તત્વ માં સહસયોજક સંયોજન બનાવવાની વૃતિ છે.
- 8View Solutionનીચેનામાંથી કયો પદાર્થ જ્યારે પાણીમાં ઓગળી જાય છે ત્યારે કોઈ દ્રાવણ બનાવે છે જે બિન-વાહક છે?
- 9View Solutionનીચેનામાંથી કોની દ્વિધ્રુવીય ચાકમાત્રા શૂન્ય છે?
- 10View Solutionનાનામાં નાનો બંધકોણ શેમાં જોવા મળે છે ?
