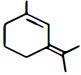
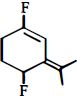
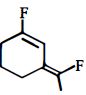
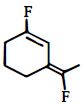
નીચેનામાંથી મહતમ ધ્રુવીય સંયોજન કયું છે?
JEE MAIN 2018, Diffcult
c
Among the substituents attached to the given compounds, fluorine has maximum electronegativity. So it will push electron pair towards it self. In option \((b),\) the two \(F\) groups are attached opposite to each other thus net dipole moment will cancel each other and reduce its polarity. In option \((d),\) the \(F\) groups are attached in slightly opposite direction thus this also decreases its polarity. But in option \((c),\) the compound has the two \(F\) groups along same direction. Thus net dipole moment will increase in this direction thus it will have maximum polarity. Thus the compound in option \((c)\) has maximum polarity
Among the substituents attached to the given compounds, fluorine has maximum electronegativity. So it will push electron pair towards it self. In option \((b),\) the two \(F\) groups are attached opposite to each other thus net dipole moment will cancel each other and reduce its polarity. In option \((d),\) the \(F\) groups are attached in slightly opposite direction thus this also decreases its polarity. But in option \((c),\) the compound has the two \(F\) groups along same direction. Thus net dipole moment will increase in this direction thus it will have maximum polarity. Thus the compound in option \((c)\) has maximum polarity
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના પૈકી ક્યુ વિધાન સાચુ નથી ?
- 2View Solutionલોથર મેયરના વક્રમાં ક્યા તત્વો ટોચ પર હતા ?
- 3નીચે બે વિધાનો આપેલા છે. એકને વિધાન $(A)$ તરીકે લેબલ કરેલ છે અને બીજા ને કારણ $(R)$ તરીકે લેબલ કરેલ છે.View Solution
વિધાન $(A)$ :$O^{2-}$ અને $Mg ^{2+}$ ની આયનિક ત્રિજ્યાઓ સમાન છે.
કારણ $(R)$ : બંને $O ^{2-}$ અને $Mg ^{2+}$ સમઈલેક્ટ્રોનીય સ્પીસિઝો છે.
ઉપરોક્ત વિધાનોના સંંદર્ભે, નીચે આપેલા વિકલ્પમાંથી યોગ્ય ઉત્તરની પસંદગી કરો.
- 4View Solutionનીચેનામાંથી કયા કદના ઘટતા ક્રમમાં ગોઠવાય છે?
- 5View Solutionઆયનીકરણની એન્થાલપી વિશે નીચેનામાંથી કયું વિધાન યોગ્ય છે?
- 6ત્રણ તત્વો $X , Y$ અને $Z$ એ આવર્ત કોસ્ટક ના $3^{ rd }$ આવર્ત માં છે . $X$, $Y$ અને $Z$ ના ઓકસાઈડ અનુક્રમે બેઝિક , ઉભયગુણધર્મી અને એસિડિક છે $X , Y$ અને $Z$ નો સાચો આણ્વિય નંબર નો શું હશે ?View Solution
- 7View Solutionઆપેલ તત્વોની ધાત્વીક પ્રકૃતિનો સાચો ક્રમ નીચે આપેલામાંથી કયો દર્શાવે છે.
- 8ઓક્સિજન અને સલ્ફરની બીજી ઇલેક્ટ્રોન પ્રાપ્તિ એન્થાલ્પી ($kJ\, mol^{-1}$ માં) અનુક્રમે કેટલી છે?View Solution
- 9$B, P, S$ અને $F$ ની પ્રથમ આયનીકરણ એન્થાલ્પીનો વધતો ક્રમ કયો હશે? (સૌથી ઓછી પ્રથમ).View Solution
- 10ઇલેક્ટ્રોનિક રચના ધરાવતું તત્વ $[Kr]4d^{10} 4f^{14} ,5s^25p^6,6s^2$ નું કોનું છેView Solution