નીચેની પ્રક્રિયા કઈ પ્રક્રિયા છે?
$C{H_2} = CH - C{H_3} + HBr \to C{H_3}CHBr - C{H_3}$
AIPMT 1996, Medium
b
In this reaction, $HBr$ undergoes heterolytic fission as
In this reaction, $HBr$ undergoes heterolytic fission as
$HBr \rightarrow H ^{+}+ Br ^{-}$
Therefore, this reaction show electrophilic addition reaction.
$CH _2= CH - CH _3+ HBr \longrightarrow CH _3-\stackrel{\oplus}{ C } H - CH _3 \stackrel{ Br ^{-}}{\longrightarrow} CH _3- CHBr - CH _3$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$C{H_3}\, - \,\,CH\,\, = \,C{H_2}\,\xrightarrow{{NOCl}}$ નીપજે, તો નીપજ શું છે ?View Solution
- 2View Solutionએક કાર્બનિક પદાર્થના ઓઝોનોલિસીસથી એક નીપજ ફોર્માલ્ડિહાઇડ મળે છે. જે તેમાં શાની હાજરી પૂરવાર કરે છે ?
- 3રૂપાંતર માટે જરૂરી પ્રક્રિયક જણાવોView Solution
$Ph - C \equiv C - Ph \to $
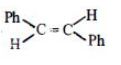
- 4View Solutionજે આલ્કિન ભૌમિતિક સમઘટકતા દર્શાવે છે તે ...... છે.
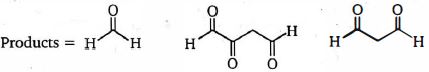
- 5View Solutionનીચેના ત્રણ ઉત્પાદનો આપવા માટે ટ્રાઇન ને એસીટીકએસિડમાં ઝીંક પછી ઓઝોન દ્વારા સારવાર આપવામાં આવે છે. તો ટ્રાઈન ની રચના શું છે?
- 6$C _{4} H _{8}$ અણુસૂત્ર સાથે ના બે સમધટકો $'A'$ અને $'B'$ની એસિડિક માધ્યમમાં $KMnO _{4}$ સાથે રિડક્શન પ્રક્રિયા કરતા જુદ્દી જુદી નીપજો પ્રાપ્ત થાય છે. સમઘટક $'A'$ ની $KMnO _{4} / H ^{+}$સાથે પ્રક્રિયા કરતાં પરિણામ સ્વરૂપે એક વાયુના ઉભરા અને એક કિટોન મળે છે. તો સંયોજન $'A'$ શોધો.View Solution
- 7નીપજ $(A)$ શું હશે ?View Solution
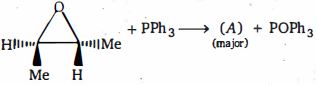
- 8View Solutionનીચેની પ્રકિયામાંથી કઈ સંભવિત મુખ્ય નીપજ છે ?
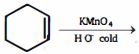
- 9નીપજ $(C)$ શું હશે ?View Solution
- 10View Solutionનીચેનામાંથી કયું એક સૌથી વધુ ક્રિયાશીલ છે જે ઈલેકટ્રો નાઈટ્રેશન તરફ રહે છે ?
