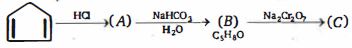
રૂપાંતર માટે જરૂરી પ્રક્રિયક જણાવો
$Ph - C \equiv C - Ph \to $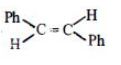
JEE MAIN 2014, Diffcult
c
With Lithium in liquid ammonia, trans-alkene is almost an exclusive product.
With Lithium in liquid ammonia, trans-alkene is almost an exclusive product.
\(Ph-C\equiv C-Ph\xrightarrow[Brich\,reduction]{Li\,in\,liq.N{{H}_{3}}}\begin{matrix}
^{Ph} \\
_{H} \\
\end{matrix}\begin{matrix}
\text{ }\!\!\backslash\!\!\text{ } \\
/ \\
\end{matrix}C=C\begin{matrix}
/ \\
\text{ }\!\!\backslash\!\!\text{ } \\
\end{matrix}_{Ph}^{H}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionબતાવેલ તમામ હાઇડ્રોકાર્બન ખૂબ નબળા એસિડ્સ છે. એક જોકે અન્ય કરતા વધુ એસિડિક છે. કયું એક સૌથી મજબૂત એસિડ છે
- 2નીચે આપેલ પ્રક્રિયામાં $X$ એ શું હશે?View Solution
$C{H_3}C{H_2}CH = CHC{H_3}$ $\xrightarrow{X}C{H_3}C{H_2}COOH\, + \,C{H_3}COOH$
- 3View Solutionનીચેની પ્રકીયામાંથી સાચી નીપજ કઈ છે ?
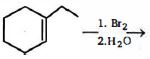
- 4$\mathrm{CH}_3 \mathrm{COONa}$ અને $\mathrm{C}_2 \mathrm{H}_5 \mathrm{COONa}$ ના મિશ્રગના વિદ્યુત વિભાજનની કેટલા આલ્કેન મળશે.View Solution
- 5View Solutionનીચે આપેલા બંધારણમાંથી કયો પ્રકૃતિમાં એરોમેટિક છે?
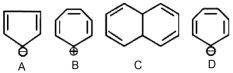
- 6પેરોક્સાઇડની હાજરીમાં પ્રોપિનની $HBr$ સાથેની પ્રક્રિયા શુ આપશે ?View Solution
- 7View Solutionપેરોક્સાઇડની હાજરીમાં, હાઇડ્રોજન ક્લોરાઇડ અને હાઇડ્રોજન આયોડાઇડ આલ્કીન્સમાં પ્રતિ-માર્કોવનિકોવ યોગશીલ આપતા નથી કારણ કે
- 8${H_2}\,C = \,\,CH{(C{H_2})_6}\, - \,\,C{H_3}\,\mathop {\xrightarrow{{peroxide}}}\limits_{HBr} \,\,$ પ્રક્રીયાની નીપજ શું હશે ?View Solution
- 9$CH_3Cl$ સાથે બેન્ઝિન નિર્જળ $AlCl_3$ની હાજરીમાં પ્રક્રિયા કરીને શું બનાવે છે?View Solution
- 10View Solutionપ્રક્રિયાની અંતિમ નીપજ કઈ છે ?