(b) The formation of \(n-\)propyl bromide in presence of peroxide can be explained as follows.
Step\(-1:\) Peroxide undergo fission to give free radicals \(R - O - O - R \to 2 - R - \dot O\)
Step\(-2 :\) \(HBr\) combines with free radical to form bromine free radical \(R - \dot O + HBr \to R - OH + B\dot r\)
Step\(-3 :\) \(B\dot r\) attacks the double bond of the alkene to form a more stable free radical
Step\(-4 :\) More stable free radical attacks the \(HBr\)
\(C{H_3} - \dot CH - C{H_2} - Br + HBr \to \mathop {C{H_3}C{H_2}C{H_2}Br}\limits_{{\rm{n - propyl bromide}}} + B\dot r\)
Step\(-5 :\) \(B\dot r + B\dot r \to B{r_2}\)
\(C{H_3}CH = C{H_2} + Br \to \mathop {C{H_3} - CH - C{H_2}Br}\limits_{(more\,stable)} \)
\(C{H_3}CH = C{H_2} + Br \to \mathop {\begin{array}{*{20}{c}}
{Br} \\
| \\
{C{H_3} - CH - C{H_2}}
\end{array}}\limits_{(less\,stable)} \)
Download our appand get started for free
Similar Questions
- 1બેન્ઝિનની સાંદ્ર $HNO_3$ અને સાંદ્ર $H_2SO_4$ ના મિશ્રણ સાથે પ્રક્રિયા કરી ત્યારબાદ $Cl_2 / FeCl_3$ સાથેની પ્રક્રિયા ... આપે છે.View Solution
- 2View Solutionનીચેનામાંથી કયું એ વધારે સ્થાયી રીતે ગોઠવણ થશે નહીં
- 3પ્રોપેન $CH_3CH = CH_2$ એ ઓક્સિડેશન દ્વારા $1$ -પ્રોપેનોલ માં રૂપાંતર પામે છે .તો જણાવો કે ઉપરના રૂપાંતરણને અસર કરવા માટે નીચેનામાંથી કયા પ્રકીયકની જોડ આદર્શ છે?View Solution
- 4પ્રકીયક $A$ શું હોઈ શકે ?View Solution
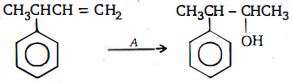
- 5View Solutionઇથેનનો આકાર........ છે.
- 6નીચે બે વિધાનો આપેલા છે.View Solution
વિધાન $I:$ ટ્રોપોલોન એ એક એરોમેટિક સંયોજન છે અને તે $8 \pi$ ઇલેકટ્રોનો ધરાવે છે.
વિધાન $II:$ ટ્રોપોલોન માં $ > C = 0$ સમૂહ ના $\pi$ ઈલેકટ્રોનો એ એરોમેટિકતામાં સંકળાયેલા છે.
ઉપરના વિધાનો ના સંદર્ભમાં,નીચે આપેલા વિકલ્પોમાંથી સાચો જવાબ પસંદ કરો.
- 7View Solutionનીચેનામાંથી એરોમેટિક કોણ નથી ?
- 8આઇસોબ્યુટીન + $HBr \rightarrow............$............View Solution
- 9$CH_3C \equiv C-H$ ની $CH_3MgX$ સાથેની પ્રક્રિયાથી શું મળે ?View Solution
- 10View Solutionઆપેલ આલ્કિનના હાઇડ્રોબોરેશન -ઑક્સિડેશન પર પ્રાપ્ત નિપજો શું છે?