પ્રક્રિયા $2Al{(s)}+6HCl{(aq)} \rightarrow 2Al^{^{3+}}{(aq)}+6Cl^-{(aq)} +3H_2{(g)}$ માં .........
AIEEE 2007, Diffcult
a
\(6\; moles\) of \(\mathrm{HCl}(\mathrm{aq})\) produces \(=3\) moles of \(\mathrm{H}_{2}(\mathrm{g})\)
\(6\; moles\) of \(\mathrm{HCl}(\mathrm{aq})\) produces \(=3\) moles of \(\mathrm{H}_{2}(\mathrm{g})\)
\(\therefore \;1 \;mole\) of \(\mathrm{HCl}(\mathrm{aq})\) produces \(=\frac{3}{6}\) moles of \(\mathrm{H}_{2}(\mathrm{g})=\frac{1}{2}\; moles\) of \(\mathrm{H}_{2}(\mathrm{g})\)
From avogadro hypothesis we know at \(STP \;1\; mole\) of any as occupies \(22.4 \mathrm{\,L}\)
\(\therefore \frac{1}{2}\) moles of \(\mathrm{H}_{2}(\mathrm{g})\) occupies \(=\frac{1}{2} \times 22.4=11.2 \mathrm{\,L}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1ચોર પાસેથી $19.7$ કિ.ગ્રા. સોનું જપ્ત કરવામાં આવ્યું તો તેની પાસેથી સોનાના કેટલા પરમાણુઓ જપ્ત કરવામાં આવેલા હશે? ($Au$ $= 197$ ગ્રામ/મોલ)View Solution
- 2$Cu + HNO_3$ $\rightarrow$ $Cu(NO_3)_2 + NO_2 + H_2O$ એસિડિક માધ્યમમાં આપેલ પ્રક્રિયા સંતુલિત કર્યા પછી નિપજો તરફની બાજુએ નાઈટ્રોજન પરમાણુની સંખ્યા, પાણીના અણુઓની સંખ્યા અને વીજભાર અનુક્રમે.....View Solution
- 3જો એક રોકેટ એ બળતણ $\left( C _{15} H _{30}\right)$ અને પ્રવાહી ઓક્સિજન પર ગતિ (દોડે) કરે છે.જરૂરી ઓક્સિજનનું વજન અને બળતળ ના દરેક લિટરે મુક્ત થતો $CO_{2}$ અનુક્રમે શોધો:(આપેલ:બળતળની ઘનતા $0.756\,g/ml$ છે.)View Solution
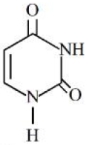
- 4નીચે આપેલ બંધારણ સાથે $RNA$ માં હાજર યુરેસીલ બેઇઝ છે. યુરિસિલમાં $N$ ના $\%............$ છે.View Solution
મોલર દળ $N =14\,g\,mol ^{-1} ; O =16\,g\,mol ^{-1} ; C =12\,g\,mol ^{-1} ; H =1\,g\,mol ^{-1}$;
- 5$+ 2$ સંયોજકતા ધરાવતી ધાતુનો તુલ્યભાર જો $12$ હોય, તો તે ધાતુના ઑક્સાઇડનો અણુભાર (આણ્વીય દળ).......થાય.View Solution
- 6$7\,g$ નાઇટ્રોજન અને $8\,g$ ઓક્સિજન ધરાવતા વાયુમિશ્રણ માટે બંને ઘટકોના મોલ-અંશ માટે સારો વિકલ્પ પસંદ કરો.View Solution
- 7$26$ મિલી $CO_2$ ને ગરમ કોલસા પરથી પસાર કરતા ઉદ્ભવતા $CO$ નું કદ એ .......... $\mathrm{ml}$View Solution
- 8$8\, {~g}$ સોડિયમમાં અણુઓની સંખ્યા ${x} \times 10^{23}$ છે. ${x}$નું મૂલ્ય $......$ છે. (નજીકના પૂર્ણાંકમાં)View Solution
$\left[\right.$ આપેલ છે $: {N}_{{A}}=6.02 \times 10^{23}\, {~mol}^{-1}$ ,${Na}$નું આણ્વીય દળ $=23.0\, {u}]$
- 9જો $LPG$ સિલીન્ડર એ બ્યુટેન અને આઈસોબ્યુટેનનું મિશ્રણ ધરાવે છે. તેના $1$ કિગ્રાના દહન માટે જરૂરી ઓક્સિજનનું મુલ્ય કેટલા .............. $\mathrm{kg}$ થાય ?View Solution
- 10પ્રક્રિયામાં $N_2 + 3H_{2} → 2NH_3$, માં કદનો ગુણોત્તર $N_2$, $H_2$ અને $NH_3$ $1 : 3 : 2$ છે. આ કયા નિયમની સમજૂતી આપે છે ?View Solution
