પ્રક્રિયા $A(g) + B(g) \to C(g) + D(g)$ માટે $298\,K$ પર $\Delta H^o$ and $\Delta S^o$ અનુક્રમે $-29.8\,\,kJ\, mol^{-1}$ અને $-0.100\,\,kJ\,K^{-1}$ $mol^{-1}$ છે $298\,K$ પર પ્રક્રિયાનો સંતુલન અચળાંક જણાવો.
JEE MAIN 2016, Diffcult
c
Given $\Delta {H^o} = - 29.8\,kJ\,mo{l^{ - 1}}$
Given $\Delta {H^o} = - 29.8\,kJ\,mo{l^{ - 1}}$
$\Delta {S^o} = - 1.00\,kJ\,{K^{ - 1}}$
From the equation
$\Delta G = \Delta {H^o} - T\Delta {S^o} = - 29.8 - (298 \times - 0.100)$
$ = - 29.8 + 29.8 = 0$
Now, $\Delta {G^o} = - 2.303\,RT\,\log \,{K_{eq}}$
$0 = - 2.303\,RT\,\log \,{K_{eq}}$
$\therefore {K_{eq}} = 1$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
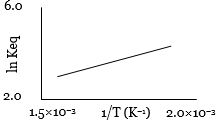
- 1એક પ્રક્રિયા માટે $\ln K_{eq}$ વિરુદ્ધ તાપમાનના વ્યસ્તનો આલેખ નીચે મુજબ છે. તો પ્રક્રિયા ........ હશે.View Solution
- 2$298\,K$ એ મિથેન $CH_{4(g)}$ ની પ્રમાણિત નિર્માણ એન્થાલ્પી $(\Delta _fH^o) -74.8 \,kJ$ મોલ$^{-1}$ છે. તો $C-H$ બંધ નિર્માણ માટેની સરેરાશ ઉર્જા માપવા વધારાની માહિતી કઈ છે ?View Solution
- 3જ્યારે $3$ મોલ $Ar_{(g)}$ વાયુ અચળ દબાણે $229$ જૂલ ઉષ્મા આપવામાં આવે છે. તો નમૂનાનું તાપમાન $2.55$ કેલ્વિન વધે છે. વાયુની અચળ તાપમાને અને અચળ કદે મોલર ઉષ્મા ક્ષમતાની ગણતરી કરો.View Solution
- 4$Ag_2O_{(s)} \rightarrow 2Ag_{(s)} + 1/2 O_{2(g)}$ પ્રક્રિયા માટે $\Delta H$ નું મુલ્ય $= 30.56 \,KJ $ મોલ$^{-1}$ છે અને $\Delta S$ = $66 \,JK$$^{-1}$ મોલ$^{-1}$ છે. તો ......$K$ તાપમાને પ્રક્રિયા માટે મુક્ત ઉર્જાનો ફેરફાર શુન્ય થશે ?View Solution
- 5View Solutionસમોષ્મી વિસ્તરણ કરતા સમતાપી પ્રકમમાં વાયુના દબાણના ઘટાડા સાથે કદનો વધારો વધારે હોય છે, કારણ કે .............
- 6View Solutionનીચેના પૈકી કઇ પ્રક્રિયામાં એન્ટ્રોપી વધે છે ?
- 7View Solutionપ્રતિવર્તીં સમતાપી પ્રક્રમમાં આંતરિય ઊર્જાનો ફેરફાર ...... થાય છે.
- 8ગીબ્સ મુક્ત ઊર્જા ${(g)}$ માટે નીચેના પૈકી કયું સૂત્ર સાચું છે ?View Solution
- 9નીચેની માહિતી પરથી નાઇટ્રિક ઓક્સાઇડની સર્જન એન્થાલ્પી કેટલા .......$kJ\,mo{l^{ - 1}}$ થશે ?$N{O_{\left( g \right)}} + C{O_{\left( g \right)}} \to 1/2{N_{2\left( g \right)}} + C{O_{2\left( g \right)}};\Delta {H^o} = - 372.2\,kJ/mol$View Solution
$\Delta H_f^o\left( {CO} \right) = - 110.5\,kJ\,mo{l^{ - 1}};$
$\Delta H_f^o\left( {C{O_2}} \right) = - 393.5\,kJ\,mo{l^{ - 1}}$
- 10$H-H,Br-Br$ અને $H-Br$ બંધની બંધઊર્જા અનુક્રમે $433,192$ અને $364\, kJ\,mol^{-1}$ હોય તો સમીકરણ ${H_2}(g) + B{r_2}(g) \to 2HBr(g)$ માટે $\Delta {H^o}$ નું મૂલ્ય કેટલા .....$kJ$ હશે ?View Solution
