પ્રક્રિયા $CH_3Br+Nu^- \rightarrow CH_3 -Nu + Br^-$ માટે કેન્દ્રઅનુરાગી $(Nu^-)$ $A$ થી $D$ ના પ્રક્રિયા દરનો ધટતો ક્રમ જણાવો
$[Nu^-=(A) \,PhO^-, (B)\,AcO^-, (C)\, HO^-, (D)\, CH_3O^-]$
AIEEE 2006, Diffcult
c
The stronger the acid, the weaker the conjugate base formed.
The stronger the acid, the weaker the conjugate base formed.
The acid character follows the order:
\(-C H_{3} C O O H>C_{6} H_{5} O H>H_{2} O>C H_{3} O H\)
The basic character will follow the order
\(-C H_{3} C O O^{-} < C_{6} H_{5} O^{-} < O^{-} H < C H_{3} O\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઆપેલ અણુની સૌથી સ્થાયી પ્રમાણભૂત રચના કઈ છે:
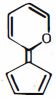
- 2View Solutionસૂર્યપ્રકાશમાં એલિવેટેડ તાપમાને ટોલ્યુઇન અને ક્લોરિનની પ્રક્રિયામાં રચાયેલી મધ્યવર્તી શું રજૂ કરે છે?
- 3નીચે આપેલ કાર્બોકેટાયનનો સ્થાયીતાનો ક્રમ કયો છે :View Solution
$(I)\,\,C{H_2} = CH - \mathop C\limits^ \oplus {H_2}$
$(II)\,\,C{H_3} - CH_2 - \mathop C\limits^ \oplus {H_2}$
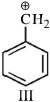
- 4View Solutionકઈ જોડીમાં બીજું આયન પ્રથમ કરતા વધુ સ્થાયી છે?
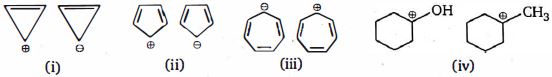
- 5View Solutionનીચેનામાંથી કયા એકબીજાના સંસ્પંદનને ગુંજારતું નથી?
- 6સંક્રમણની સ્થિતિને ક્રમ આપો ક્રમમાં નીચેના પ્રતિક્રિયા પગલાં દરમિયાન થાય છેView Solution
વધતી સ્થિરતા ( ઓછા માં ઓછી થી વધારે સ્થાયી)$1. \,{H_3}C - \mathop O\limits^ + {H_2} \to CH_3^ + + {H_2}O$
$2.\,{(C{H_3})_3}C - \mathop O\limits^ + {H_2} \to {(C{H_3})_3}{C^ + } + {H_2}O$
$3.\,{(C{H_3})_2}CH - \mathop O\limits^ + {H_2} \to {(C{H_3})_2}C{H^ + } + {H_2}O$
- 7View Solutionનીચેનામાંથી કયો પદાર્થ વધુ બેઝિક છે ?
- 8View Solutionકાર્બએનાયન ની સ્થિરતા નો સાચો ક્રમ શોધો.
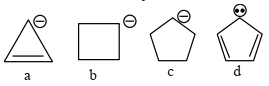
- 9View Solutionએસિડ પ્રબળતા ઓછી થવાના ક્રમમાં નીચેના સંયોજનો ક્રમ આપો મોટાભાગે એસિડિક થી ઓછામાં ઓછું એસિડિક)
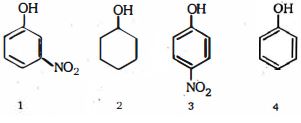
- 10View Solutionઇલેક્ટ્રોન અનુરાગી એરોમેટિક વિસ્થાપનમાં નીચેના સંયોજનો પૈકી ક્યુ સૌથી વધુ પ્રતિક્રિયાત્મક છે ?
