$SO_2$ પરમાણુ સંબંધિત સાચું વિધાન છે?
Medium
d
\(\mathrm{SO}_{2}\) molecule has one \(\mathrm{p} \pi-\mathrm{p} \pi\) bond and one \(\mathrm{p} \pi-\mathrm{d} \pi\) bond
\(\mathrm{SO}_{2}\) molecule has one \(\mathrm{p} \pi-\mathrm{p} \pi\) bond and one \(\mathrm{p} \pi-\mathrm{d} \pi\) bond
\(\mathrm{SO}_{2}=6+6(2)=18 \mathrm{e}-16 \mathrm{e}=2 \mathrm{e} \rightarrow 1\) lone pair
\(\rightarrow \mathrm{G} . \mathrm{S}=[\mathrm{Ne}] 3 \mathrm{s}^{2} 3 \mathrm{p}^{4}\)
2 Unpaired electron \(1^{\text {st }}\) E.S. \(=[\mathrm{Ne}] 3 \mathrm{s}^{2} 3 \mathrm{p}^{3} 3 \mathrm{d}^{1}\)
4 unpaired electron \(\mathrm{O} \rightarrow 1 \mathrm{s}^{2} 2 \mathrm{s}^{2} 2 \mathrm{p}^{4}\)
\(\rightarrow\) Geometry of \(\mathrm{SO}_{2}\) is BENT/ANGULAR
\(\rightarrow\) one \(\mathrm{p} \pi-\mathrm{p} \pi\) and one \(\mathrm{p} \pi-\mathrm{d} \pi\) Bond
\(\rightarrow 2 \pi\) Bonds
\(\rightarrow 1\) lone pair on the central atom.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$IF_5 , I_3^-$ અને $I^+_3$માં કેન્દ્રીય આયોડિન અણુનું સંકરણ અનુક્રમે છે,View Solution
- 2નીચે પૈકી કયું સંયોજન જેમાં $X - Cl$ બંધ સાથે સંકળાયેલ સૌથી વધુ આયનીય ગુણધર્મ છેView Solution
- 3જલીય દ્રાવણમાં એક દ્વિસંયોજક આયનની (પરમાણુ ક્રમાંક $29$) સ્પીન ફકત ચુંબકીય ચાકમાત્રા .... $BM$ છે.View Solution
- 4View Solutionહાઇડ્રોજન બંધ ની શક્તિ કોની વચ્ચે હોય છે?
- 5સ્પિન-માત્ર ${B}_{2}^{+}$ આયનનું ચૂંબકીય ચાકમાત્રાનું મૂલ્ય $......\,\times 10^{-2} \,{BM}$ છે. (નજીકના પૂર્ણાંકમાં)View Solution
[આપેલ છે : $\sqrt{3}=1.73$ ]
- 6View Solutionનીચેનામાંથી કોણ ધ્રુવીય છે ?
- 7$PCl_3Br_2$ ભૌમિતિક સમઘટકતા દર્શાવે છે, જેના ભૌમિતિક સમઘટકો નીચે મુજબ છે. તે પૈકી કોણ દ્વિધ્રુવ ચાકમાત્રા ધરાવતો(તા) નથી ?View Solution
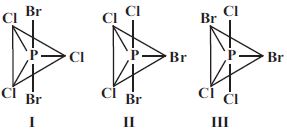
- 8નીચે આપેલી આયનિય સંયોજનોની જોડીમાં સંયોજનના કયા સમૂહમાં લેટિસની ઊર્જા વધારે છે?View Solution
$(i)\, KCl$ અથવા $MgO$ $(ii) \,LiF$ અથવા $LiBr$ $(iii)\, Mg_3N_2$ અથવા $NaCl$
- 9View Solutionઓક્સિજન અણુમાં જોડાયેલા ઇલેક્ટ્રોનની સંખ્યા કેટલી છે ?
- 10$SF_2Cl_2$ અણુનો આકાર . ... છે.View Solution
