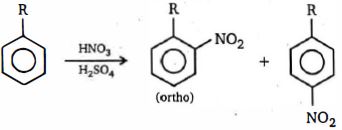
$(a)$ The steric demand of $H^{\ominus}$ is, however extremely small, and when attack on $C_6H_5Y$ is by any other electrophile, $E^{\oplus}$ , which will necessarily be larger, there will be increasing interaction between $E$ and $Y$ in the transition state for attack at the position $o-$ to $Y (57 \,b, R = E)$ as attacking electrophile and substituent increase in size ; there can be no such interaction in the transition state for $p-$ attack $(57a, R = E)$. This will be reflected in an increasing $\Delta \,G^+$ for $o-$ attack, a consequently slower reaction, and the relative proportion of $o-$ product will thus fall as the size of $E$ and / or $Y$ increase. This is illustrated by the falling $f_{o^-} /f_{p^-}$ ratios which are observed for the nitration of alkylbenzenes $(Y ---CH_3 \to CMe_3)$ under comparable conditions ;
Increase in size of $Y$ $\begin{gathered}
\downarrow \hfill \\
\downarrow \hfill \\
\downarrow \hfill \\
\downarrow \hfill \\
\downarrow \hfill \\
\end{gathered} $ $\begin{array}{*{20}{c}}
Y&{\% \,\,o - }&{\% \,p\, - } \\
{C{H_3}}&{58}&{37} \\
{C{H_2}Me}&{45}&{49} \\
{CHM{e_2}}&{30}&{62} \\
{CM{e_3}}&{16}&{73}
\end{array}$
Download our appand get started for free
Similar Questions
- 1ફ્રિડલ ક્રાફટસ પ્રક્રિયામાં નિર્જળ $AlCl_3$ નું કાર્ય ........ છે.View Solution
- 2નીચેની પ્રક્રિયાઓના ક્રમમાં એક જ સમતલમાં $'C$'માં અણુઓની મહત્તમ સંખ્યા હાજર છેView Solution
$A \xrightarrow[ { Cu\; tube }]{\text { Redhot }}\mathrm{B} \xrightarrow[ Anhydrous AlCl_3]{\mathrm{CH}_{3} \mathrm{Cl}(1 \mathrm{eq}} \mathrm{C}$
($A$ એ સૌથી ઓછું પરમાણ્વીય વજન ધરાવતું આલ્કાઇન છે)
- 3View Solutionનીચેનામાંથી કઈ પ્રક્રિયામાં, માર્કોવનિકોફના નિયમનું ઉલ્લંઘન થાય છે?
- 4$\begin{array}{*{20}{c}}View Solution
{M{e_2}CH - CH - Me} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,\,\,\,} \\
{\,\,\,\,\,\,\,\,OH}
\end{array}\xrightarrow[{350{\,^o}C}]{{A{l_2}{O_3}}}(A)\xrightarrow[{(ii)\,AgOH}]{{(i)\,HI}}(B)$ઉપરોક્ત પ્રકિયા માં નીપજ $(B)$ શું હશે ?
- 5$Br_2$ થી ટ્રાન્સ- $2$ -બ્યુટીન માં ઉમેરવું એક ઉત્પાદન આપે છે જે કયું છે ?View Solution
- 6View Solutionફ્રિડલ-ક્રાફટ્સ પ્રક્રિયામાં ટોલ્યુઇન...... વડે બનાવી શકાય.
- 7View Solutionએરોમેટિક સંયોજનોના નાઈટ્રેશન પર નીચેના વિધાનોમાં કયુ ખોટું છે?
- 8નીચે બતાવેલ $3,3$ - ડાયમિથાઇલ - $1$ -બ્યુટીન ના વધારાને ધ્યાનમાં લો.નિરીક્ષણ કરેલ નીપજ ની રચના માટે શ્રેષ્ઠ પદ્ધતિઓની સમજૂતી શું છે?View Solution
- 9View Solutionકઇ પ્રક્રિયામાં આલ્કેન અને આલ્કીન બંને મળે છે ?
- 10નીચેની પ્રક્રિયામાં $M$ અને $R$ અનુક્રમે ........ હશે.View Solution
$C{H_2} = C{H_2}\xrightarrow[{oxid}]{{Hypochloro}}$ $M\xrightarrow{R}\begin{array}{*{20}{c}}
{C{H_2} - OH} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_2} - OH}
\end{array}$
