Rate of reaction (ROR)
$=-\frac{1}{2} \frac{\Delta\left[\mathrm{N}_2 \mathrm{O}_5\right]}{\Delta \mathrm{t}}=\frac{1}{4} \frac{\left[\mathrm{NO}_2\right]}{\Delta \mathrm{t}}=\frac{\Delta\left[\mathrm{O}_2\right]}{\Delta \mathrm{t}}$
$\mathrm{ROR}=-\frac{1}{2} \frac{\Delta\left[\mathrm{N}_2 \mathrm{O}_5\right]}{\Delta \mathrm{t}}=-\frac{1}{2} \frac{(2.75-3)}{30} \mathrm{molL}^{-1} \mathrm{~min}^{-1}$
$\mathrm{ROR}=-\frac{1}{2} \frac{(-0.25)}{30} \mathrm{molL}^{-1} \mathrm{~min}^{-1}$
$\text { ROR }=\frac{1}{240} \mathrm{molL}^{-1} \mathrm{~min}^{-1}$
$\text { Rate of formation of } \mathrm{NO}_2=\frac{\Delta\left[\mathrm{NO}_2\right]}{\Delta \mathrm{t}}=4 \times \mathrm{ROR}$
$=\frac{4}{240}=16.66 \times 10^{-3} \mathrm{molL}^{-1} \mathrm{~min}^{-1} \simeq 17 \times 10^{-3}$
Download our appand get started for free
Similar Questions
- 1પદાર્થ $A$ અને $B$ વચ્ચેની પ્રક્રિયા માટેની વેગનિયામ નીચે મુજબ છે. વેગ $= K[A]^n[B]^m $ જો $A$ નું સાંદ્રણ બમણું કરવામાં આવે તથા $B$ ની સાંદ્રતા અડધી કરવામાં આવે તો નવા વેગ એ મૂળવેગ વચ્ચેનો ગુણોત્તર કેટલો થશે ?View Solution
- 2View Solutionપ્રથમ ક્રમની પ્રક્રિયા માટે સાચું સમીકરણ લખો.
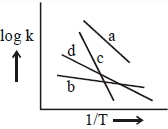
- 3ચાર જુદી જુદી પ્રક્રિયાઓ માટે વેગ અચળાંક વિરુદ્ધ $\frac{1}{\mathrm{T}}$ ના નીચેના આલેખ ધ્યાનમાં લો. તો આ પ્રક્રિયાઓની સક્રિયકરણ ઊર્જાઓ માટે નીચેના પૈકી ક્યો ક્રમ સાચો છે ?View Solution
- 4પ્રક્રિયા $A \to B$ પ્રથમ ક્રમની ગતિકીને અનુસરે છે. $A$ ના $0.8$ મોલમાંથી $B$ ના $0.6$ મોલ ઉત્પન્ન કરવા $1$ કલાક લાગે છે. તો $A$ ના $0.9$ મોલમાંથી $B$ ના $0.675$ મોલ ઉત્પન્ન કરવા .......... કલાક લાગશે .View Solution
- 5સાદી રાસાયણિક પ્રક્રિયા $A \rightarrow B$ માટે પુરોગામી પ્રક્રિયાની સક્રિયકરણ ઊર્જા $E_a$ છે. તો પ્રતિગામી પ્રક્રિયાની સક્રિયકરણ ઊર્જા ............View Solution
- 6$A + B \rightarrow $ નિપજ, પ્રક્રિયા માટે પ્રક્રિયાનો દર બમણો થશે જ્યારે $A$ ની સાંદ્રતા બમણી થાય, તો દર ફરીથી બમણો થશે જ્યારે $A $ અને $ B$ ની સાંદ્રતા બમણી કરતા પ્રક્રિયાનો ક્રમ ...... થશે.View Solution
- 7View Solutionજ્યારે પ્રક્રિયાની પ્રારંભિક સાંદ્રતા બમણી કરવામાં આવે ત્યારે શૂન્ય ક્રમની પ્રક્રિયાનો અર્ધઆયુષ્ય સમય ......
- 8પ્રતિવર્તી પ્રક્રિયા $2N{O_2}\underset{{{K_2}}}{\overset{{{K_1}}}{\longleftrightarrow}}{N_2}{O_4}$ માટે $NO_2$ ના દૂર થવાનો દર....... થશેView Solution
- 9જો એક પ્રક્રિયા આર્હેનિયસના સમીકરણને અનુસરતી હોય તો $In k$ વિરૂધ્ધ $1/(RT)$ નો આલેખ સીધી રેખા આપશે, જેનો ઢાળ $(-y)$ એકમ હશે પ્રક્રિયાને સક્રિય કરવા કેટલી ઉર્જાની જરૂર પડે?View Solution
- 10ધુમ્મસના ઘટક પેરોક્સિ એસિટાઇલ નાઇટ્રેટ $(PAN)$ નુ વિઘટન પ્રથમ ક્રમની પ્રક્રિયા મુજબ પેરોક્સિ એસિટઇલ રેડિકલ અને $N{O_{2\left( g \right)}}$ માં થાય છે, જેનો અર્ધઆયુષ્ય સમય $32\,\min$ છે.View Solution
$\begin{matrix}
O\,\,\,\,\,\,\, \\
||\,\,\,\,\,\,\, \\
C{{H}_{3}}-C-OON{{O}_{2}} \\
\end{matrix}$ $\to$ $\begin{matrix}
\,\,\,\,\,\,\,\,\,\,\,O\,\,\,\,\,\,\,\, \\
\,\,\,\,\,\,\,\,\,||\,\,\,\,\,\,\, \\
C{{H}_{3}}-C-O\overset{\centerdot }{\mathop{O}}\, \\
\end{matrix}$ $ + N{O_2}$જો હવાના નમૂનામાં $PAN$ ની શરૂઆતની સાંદ્રતા $5.0 \times 10^{14}\, molecules/L$ હોય તો $1.5\, hr$ પછી સાંદ્રતા કેટલી થશે ?
