Using, first law of thermodynamics,
\( \Delta \mathrm{U}=\mathrm{Q}+\mathrm{W}, \)
\( \Delta \mathrm{U}=0: \text { Process is isothermal } \)
\( \mathrm{Q}=-\mathrm{W} \)
\( \mathrm{W}=-\mathrm{P}_{\text {ext }} \Delta \mathrm{V}: \text { Irreversible } \)
\( =-80 \times 10^3(45-30) \times 10^{-3} \)
\( =-1200 \mathrm{~J}\)
Download our appand get started for free
Similar Questions
- 1પાણીના ઉત્કલન બિંદુએ $900\,J/g$ ઉષ્માનો વિનિમય થાય છે. તો એન્ટ્રોપીમાં થતો વધારો કેટલા ......$J/K-mole$ ?View Solution
- 2View Solutionધાતુ ઓક્સાઇડને ધાતુમાં રીડકશન થવાની તરફેણમાં નીચેનામાથી કયુ છે?
- 3નીચેના પૈકી ક્યા પ્રકમ માટે $\Delta {H^o} - \Delta {G^o}$ મૂલ્ય લગભગ શૂન્ય થશે ?View Solution
- 4નીચેના સમીકરણ માટે નિયત તાપમાને $\Delta H- \Delta E$ નું મૂલ્ય કેટલુ થશે ?View Solution
${C_3}{H_8}(g) + 5{O_2}(g) \to \,\,3C{O_2}(g) + 4{H_2}O(l)$
- 5$350\,K$ પર આદર્શ વાયુ અને $4\,atm$ થર્મલ વાહક દિવાલોના $2.0\,L$ પાત્રમાં હોય છે, જે પર્યાવરણના સંપર્કમાં હોય છે.તે $4\,atm$ ના અચળ દબાણ વિરુદ્ધ સમતાપી પ્રતિવર્તી $........\,J\,K ^{-1}$ છે.(નજીકનો પૂર્ણાક) આપેલ : $R =8.314\,J\,K ^{-1}\,mol ^{-1}$.View Solution
- 6નિશ્ચિત તાપમાને $T$, ઉષ્માશોષક પ્રક્રિયા $A$ $\rightarrow$ $B$ સંપૂર્ણ પુરી થવા માટે એન્ટ્રોપી ફેરફાર .......View Solution
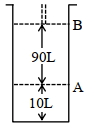
- 7આપેલ આક્રૂતિને ધ્યાનમાં લો.View Solution
$18^{\circ} \mathrm{C}$ પર, સ્થાન $A$ પર, પિસ્ટન સાથે જોડેલા (fitted) સિલિન્ડર માં આદર્શ વાયુનો $1$ $\mathrm{mol}$ રાખેલ છે. જો તાપમાન માં કોઈપણ જાતનો ફેરફાર ન કરીએ તો પિસ્ટન એ સ્થાન $B$ તરફ ખસે છે ત્યારે આ પ્રતિવર્તી પ્રક્રમ માં થયેલ કાર્ય $'x' L atm$ છે. $x=-$ ........... $L.atm$ (નજીક નો પૂર્ણાક)
[આપેલ : નિરપેક્ષ તાપમાન $={ }^{\circ} \mathrm{C}+273.15, \mathrm{R}=0.08206 \mathrm{~L} \mathrm{~atm} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$ ]
- 8કોલમ$-I$ ના રૂપાંતરણને કોલમ$-II$ માં આપેલ યોગ્ય વિકલ્પ સાથે જોડો.View Solution
કોલમ$-I$ કોલમ$-II$
$(A)\;CO_2(s)\;\to\;CO_2(g)$
$(p)$ સંક્રાંતિ માધ્યમ
$(B)\;CaCO_3(s)\;to\;CaO(s)$
$+ CO_2(g)$
$(q)$ અપરરૂપ ફેરફાર
$(C)\;2H^{\cdot}\;\to\;H_2(g)$
$(r)\;\Delta\, H \,\frac{1}{2}$ ધન છે.
$(D)\;P$ (સફેદ ધન) $\to\;P$( વાવ ધન)
$(s)\;\Delta\,S \,\frac{1}{2}$ ધન છે.
$(t)\;\Delta\, S$ ઋણ છે.
- 9$C + O_2 \rightarrow CO_2 + 94.2$ કિલોકેલરી, :$\Delta H = -94.2$ કિલોકેલરી, $H_2 + 1/2 O_2 \rightarrow H_2O + 68.3$ કિલોકેલરી : $\Delta H = -68.3$ કિલોકેલરી અને $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + 210.8$ કિલોકેલરી : $\Delta H = -210.8$ કિલોકેલરી, તો મિથેનની સર્જન ઉષ્મા $= .....$ કિલોકેલરીView Solution
- 10$27\,^oC$ તાપમાને $5$ મોલ આદર્શ વાયુ $8$ $dm^{3}$ થી $80 \,dm^{3}$ કદમાં પ્રતિવર્તીં વિસ્તરણ થાય છે. તો એન્ટ્રોપીનો ફેરફાર ......$JK ^{-1}$ થશે.View Solution
