$A _2+ B _2 \rightarrow 2 AB . \Delta H_f^0=-200\,kJ\,mol ^{-1} AB , A _2$ અને $B _2$ એ દ્રીપરમાણ્વિક અણુઓ છે. $A _2, B _2$ અને $AB$ બી બંંધ એન્થાલ્પીઓ $1:0.5:1$ના ગુણોત્તરમાં હોય તો, તો પછી $A _2$ ની બંંધ એન્થાલ્પી $.........\,kJ\,mol ^{-1}$ (નજીકનો પૂર્ણાક)
JEE MAIN 2023, Diffcult
c
\(A _2+ B _2 \rightarrow 2 AB ; \Delta H _{ r }^0=-200\,kJ\,mol ^{-1}\)
\(A _2+ B _2 \rightarrow 2 AB ; \Delta H _{ r }^0=-200\,kJ\,mol ^{-1}\)
\(\Rightarrow \Delta H _{ p }^0( AB )=-200\,kJ\,mol\,m ^{-1}\)
\(\therefore \Delta H _{ R }^0\) for reaction \(A _2+ B _2 \rightarrow 2 AB\) is \(-400\,kJ\,mol ^{-1}\)
Given: Bond Enthalpy of \(A _2, B _2\) and \(AB\) is \(1: 0.5: 1\)
Assuming bond enthalpy of \(A _2\) be \(x\, kJ\, mol { }^{-1}\)
\(\therefore\) Bond enthalpy \(B _2=0.5 \times kJ\, mol ^{-1}\)
\(\therefore\) Bond enthalpy \(AB =( x ) kJ\, mol\, m ^{-1}\)
\(-400= x +0.5 x -2 x\)
\(-400=-0.5 x\)
\(\therefore x =800\,kJ / mol\)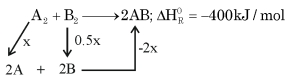
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$400\, mL$ $0.2\, M$ $H_2SO_4$ ને $600\, mL$ $0.1\, M$ $NaOH$ સાથે મિશ્ર કરવામાં આવે ત્યારે ઉત્પન્ન થતી ઉષ્માનો જથ્થો $3.43\, kJ$ છે. જો પાણીની વિશિષ્ટ ઉષ્મા $4.18\, J\, K^{-1}\,g^{-1}$ હોય, તો અંતિમ દ્રાવણના તાપમાનમાં થતો વધારો ...........$K$ થશે. (અંતિમ દ્રાવણની વિશિષ્ટ ઉષ્માક્ષમતા પાણી જેટલી ધારો)View Solution
- 2View Solutionઉષ્માશોષક પ્રક્રિયા કઈ છે?
- 3View Solutionનીચેના પૈકી ક્યુ સાચું છે ?
- 4જો કોઇ રાસાયણિક પ્રક્રિયા માટે $\Delta H = \Delta S > 0$ હોય, તો $\Delta H =$ ................View Solution
- 5$300\,K$ એ $C_6H_5COOH_{(s}), CO_{2(g)}$ અને $H_2O_{(l)}$ ની પ્રમાણિત નિર્માણ એન્થાલ્પી અનુક્રમે $-408, -393$ અને $-286\, kJ \,mol^{-1}$ છે. તો અચળ કદે બેન્ઝોઈક એસિડની દહન ઉષ્મા કેટલા .....$kJ$ થાય ?$(R = 8.31 \,J \,mol^{-1}\,K^{-1})$View Solution
- 6એન્ટ્રોપી $(S)$ ને થર્મોડાયનેમિક્સ માપદંડ ગણતા આપમેળે થતા દરેક પ્રક્રમ માટે સ્વયંભૂયિતાની શરત ........View Solution
- 7કાર્બન અને કાર્બન મોનોક્સાઇડની દહન એન્થાલ્પી અનુક્રમે $-393.5$ અને $-283\, kJ\, mol^{-1}$ છે. તો પ્રતિ મોલ દીઠ કાર્બન મોનોક્સાઇડની સર્જન એન્થાલ્પી .......$kJ$View Solution
- 8View Solutionઆદર્શ વાયુના ઉષ્મીય પ્રસરણ દરમ્યાન તેની.......
- 9$N{H_{3\left( g \right)}}$ અને ${N_2}{H_{4\left( g \right)}}$ ની પરમાણ્વિયકરણ એન્થાલ્પી અનુક્રમે $+150\, kJ\, mol^{-1}$ અને $310\, kJ\, mol^{-1}$ છે. તો $\Delta H\left( {N - N} \right)$ ........$kJ\, mol^{-1}$ થશે.View Solution
- 10પ્રબળ એસિડ અને પ્રબળ બેઇઝના તટસ્થ થવાની ઉષ્મા $57.0\,kJ\,mo{l^{ - 1}}$ છે. $HN{O_3}$ ના $0.5$ મોલ અને $KOH$ના $0.2$ મોલને મિશ્રણ કરતાં મુક્ત થતી ઉષ્મા......$kJ$ છે.View Solution
