\(4 \mathrm{gm}\) of \(\mathrm{NaOH}\) in \(100 \mathrm{L}\) sol. \(\Rightarrow 10^{-3} \mathrm{M}\) sol.
\(9.8 \mathrm{gm}\) of \(\mathrm{H}_{2} \mathrm{SO}_{4}\) in \(100 \mathrm{L}\) sol. \(\Rightarrow 10^{-3} \mathrm{M}\) sol.
Mixture : \(40 L\) of \(10^{-3} \mathrm{M} \mathrm{NaOH}\) and \(10 \mathrm{L}\) of
\(10^{-3} \mathrm{M} \mathrm{H}_{2} \mathrm{SO}_{4}\) sol.
Final Conc. of \(OH^-=\frac{10^{-3}(40 \times 1-10 \times 1 \times 2)}{40+10}=6 \times 10^{-4} \mathrm{M}\)
\(\mathrm{pOH}=-\log \left(6 \times 10^{-4}\right)\)
\(=4-\log 6=4-0.60=3.40\)
\(\mathrm{pH}=14-3.40=10.60\)
Download our appand get started for free
Similar Questions
- 1નીચે બતાવેલ બે એસિડ સામેલ નીચેની પ્રક્રિયા ધ્યાનમાં લો: ફોર્મિક એસિડ અને. $HF$View Solution
આ પ્રક્રિયા વિશે નીચેનામાંથી કયું વિધાન સાચું છે
$(A)$ પ્રક્રિયામાં ફોર્મિક એસિડ સૌથી મજબૂત બ્રોન્સ્ટેડ એસિડ છે
$(B)\, HF$ પ્રક્રિયામાં સૌથી મજબૂત બ્રોન્સ્ટેડ એસિડ છે
$(C)\, KF$ પ્રક્રિયા માં સૌથી મજબૂત બ્રોન્સ્ડ બેઇઝ છે
$(D)\, KO_2CH$ પ્રક્રિયા માં સૌથી મજબૂત બ્રોન્સ્ડ બેઇઝ છે
$(E)$ સંતુલન પ્રક્રિયા આપનારાઓની તરફેણ કરે છે
$(F)$ સંતુલન નિપજોની તરફેણ કરે છે
$(G)$ ફોર્મીક એસિડનો નબળો સનયુગ્મ બેઇઝ હોય છે
$(H)\, HF$ નબળો સયુંગ્મ બેઈઝ ધરાવે છે
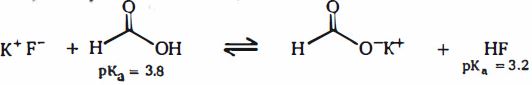
- 2સાંદ્રતા '$C$',વિયોજન અંશ ' $\alpha$ ' ના એક નિર્બળ વિદ્યુતવિભાજ્ય ( $K _{ eq }=$ સંતુલન અચળાંક) $A _2 B _3$ ના એક સાંદ્ર દ્રાવણ માટે $.........$View Solution
- 3$10^{-2}\, M\, HCN$ અને $[H^+]$ = $10^{-3}$ માટે વિયોજન અચણાંક નું મુલ્ય.....$\%$ માં શોધો.View Solution
- 4$CH_3COOH$ અને $NaOH$ ના અનુંમાપન માટે કયો સૂચક વપરાય છે ?View Solution
- 5$AgCl$ નો $K_{sp} =1.8\times 10^{-10}$ છે. તો $4 \times 10^{-3}\,M\,Ag^+$ ધરાવતા દ્રાવણમાં $AgCl$ ના અવક્ષેપન માટે જરૂરી $Cl^-$ ની સાંદ્રતા ........ થશે.View Solution
- 6જો $25\,°C$ એ $MX_2$ ક્ષાર અલ્પ દ્રવ્યનો દ્રાવ્યતા ગુણાકાર $K_{sp} = 1.0 \times 10^{-11}$ છે. તો આ તાપમાને $L^{-1}$ મોલમાં ક્ષારની દ્રાવ્યતા $= ?$View Solution
- 7એક વિશ્લેષક $pH =1$ ના $1L\,HCl$ ને $pH 2$ ના $HCl$ ના દ્રાવણ માં પરિવર્તન કરવા ઇચ્છે છે.આ મંદન કરવા માટે જરૂરી પાણીનું કદ $........\,mL$ છે.(નજીકનો પૂર્ણાક)View Solution
- 8View Solution...... સૌથી વધુ પ્રબળ લુઈસ એસિડ છે.
- 9દ્રાવણમાં ક્ષાર $AB$ની દ્રાવ્યતા નીપજ $1\times10^{-8}$ છે જેમાં $A^+$ આયનોની સાંદ્રતા $10^{-3}\, M$ છે. જ્યારે $B^-$ આયનની સાંદ્રતા કેટલી રાખવામાં આવે છે ત્યારે ક્ષારનું અવક્ષેપન થશે.View Solution
- 10હાઇડ્રાઝોઇક એસિડ $(N_3H)$ નો સંયુગ્મિત બેઇઝ = .......View Solution
