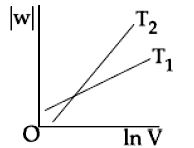
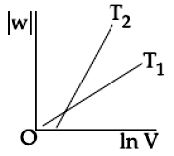
Let the gas is expanded from \(V_1\) to \(V\) at \(T_1\) and from \(V_2\) to \(V\) at \(T_2\)
\(\therefore \) At \(T_1\)
\([{W_1}] = nR{T_1}\,\ln \,\frac{V}{{{V_1}}} = nRT\,(\ln \,V - \ln \,{V_1})\)
\(Similarly\,at\,{T_2}\)
\([{W_2}] - nR{T_2}(\ln \,V - \ln \,{V_2})\)
\(\therefore \,{W_1} = nR{T_1}\ln \,V - nR{T_1}\,\ln \,{V_1}\)
\({W_2} = nR{T_2}\,\ln \,V - nR{T_2}\,\ln \,{V_2}\)
Slope of \({W_2} > \) Slope of \(W_1\)
As \(nR{T_2} > nR{T_1}({T_2} > {T_1})\)
\(\therefore \) The intercept of \(W_2\) is more negative than that of \(W_1\) because \(V_2 > V_1.\)
Download our appand get started for free
Similar Questions
- 1નિરપેક્ષ તાપમાન $T$ એ એક રાસાયણિક પ્રક્રિયા માટે પ્રમાણિત પ્રક્રિયા ગીબ્સ ઊર્જા નીચે મુજબ આપી શકાય,View Solution
${\Delta _r}{G^o} = A - BT$
જ્યાં $A$ અને $B$ શૂન્ય સિવાયના અચળાંકો છે. આ પ્રક્રિયા માટે નીચે આપેલા માંથી કયું સાચું છે?
- 2નીચેના પ્રકમો પરથી પ્રકિયા $B + D \rightarrow E + 2C$ માટે $\Delta H$ નું મૂલ્ય કેટલા ........... $\mathrm{kJ/mol}$ હશે તે જણાવો.View Solution
$\Delta H \,(kJ/mol)$ $\frac 12 A \rightarrow B$ $+150$ $3B \rightarrow 2C + D$ $-125$ $E + A \rightarrow 2D$ $+350$ - 3$(i)$ ટેકરીની ઊચાઈ $(ii)$ ટેકરીના ચડવામાં કપાતું અંતર $(iii)$ ટેકરીમાં ચડવાની ઊર્જા ફેરફાર માં કયું અવસ્થા વિધેય છે ?View Solution
- 4આપેલ આકૃતિના આધારે, સાયા વિધાન/નો ની સંખ્યા $.........$ છે.View Solution
$A.$ જથ્થામાં (બલ્કમાં) પ્રવાહી અણુ પર આકર્ષણ અને અપાકર્ષણ બળો સમાન રીતે વર્તે ત્યારે પૃષ્ઠતાણનું નિર્માણ થાય છે.
$B.$ સપાટી ઉપર હાજર અણુઓ પર અસમાન બળો પ્રવર્તમાન $(uneven\,forces)$ના કારણે પૃષ્ઠતાણ છે.
$C.$ જથ્થામાં (બલ્કમાં) અણુ પ્રવાહી સપાટી (સ્તર) પર આવતાં નથી.
$D.$ જો પ્રણાલી એ બંધ પ્રણાલી હોય તો સપાટી ઉપરના અણુઓ એ બાષ્પદબાણ માટે જવાબદાર છે.
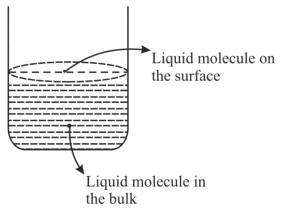
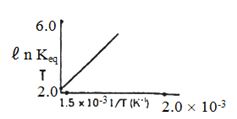
- 5પ્રક્રિયા માટે $ℓ\,n\, K_{eq}$ વિરૂદ્ધ તાપમાનનો આલેખ દોરવામાં આવે તો પ્રક્રિયા...... હોવી જોઈએ.View Solution
- 6આપેલ બંધઊર્જાના મૂલ્યો પરથી નીચેની પ્રક્રિયાની એન્થાલ્પી કેટલા ................ $\mathrm{kJ \,mol}^{-1}$ થશે ?View Solution
$H - H$ બંધઊર્જા $:\, 431.37 \,kJ\, mol^{-1}$ $C= C$ બંધઊર્જા $:\, 606.10\, kJ \,mol^{-1}$ $C - C$ બંધઊર્જા $:\, 336.49\, kJ\, mol^{-1}$ $C - H$ બંધઊર્જા $:\, 410.50\, kJ\, mol^{-1}$ પ્રક્રિયા : $\begin{array}{*{20}{c}}
{H\,\,\,\,H} \\
{|\,\,\,\,\,\,\,\,|} \\
{C = C} \\
{|\,\,\,\,\,\,\,\,\,|} \\
{H\,\,\,\,H}
\end{array}\, + \,H - H\, \to \,\begin{array}{*{20}{c}}
{H\,\,\,\,H} \\
{|\,\,\,\,\,\,\,\,|} \\
{H - C - C - H} \\
{|\,\,\,\,\,\,\,\,\,|} \\
{H\,\,\,\,H}
\end{array}\,$ - 7View Solutionમિથેનમાંથી મિથાઈલ રેડિકલની રચના કરતાં ટોલ્યુઇનમાંથી બેન્ઝાઈલ રેડિકલ બનાવવા માટે જરૂરી બંધ વિયોજન ઊર્જા એ ..... છે.
- 8પ્રબળ એસિડ અને પ્રબળ બેઈઝના તટસ્થીકરણથી પ્રતિ મોલ $H^+$ આયનોએ ઉદભવતી ઉષ્મા એ ...... હોય છે.View Solution
- 9$NCl_3$ જેવાં વિસ્ફોટક પદાર્થની $\Delta H_f^o$ નિર્માણ ઉષ્મા ...... થશે.View Solution
- 10નીચેના માંથી ક્યાં સંબંધો સાચાં છે?View Solution
$(A)$ $\Delta U = q + p \Delta V$
$(B)$ $\Delta G =\Delta H - T \Delta S$
$(C)$ $\Delta S =\frac{ q _{ rev }}{ T }$
$(D)$ $\Delta H =\Delta U -\Delta nRT$
નીચે આપેલા વિકલ્પોમાંથી યોગ્ય ઉત્તર પસંદ કરો.