The value of \(E_{M^{2}+/ M}^{\circ}\) for given metal ions are
\(E_{M n^{2}+/ M n}^{\circ}=-1.18 V\)
\(E_{C r^{2}+/ C r}^{\circ}=-0.9 V\)
\(E_{F e^{2+} / F e}^{\circ}=-0.44 V\) and
\(E_{C o^{2}+/ C o}^{\circ}=-0.28 V\)
The correct order of \(E_{M^{2+} / M}^{\circ}\) values without
considering negative sign would be
\(M n^{2+}>C r^{2+}>F e^{2+}>C o^{2+}\)
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેના પૈકી ક્યા આયન મંદ એસિડ સાથેની પ્રક્રિયાથી હાઇડ્રોજન વાયુ મુક્ત કરશે નહિ?
- 2View Solutionએસિડિફાઇડ સલ્ફેટ દ્રાવણના વિદ્યુતવિભાજય ઓક્સિડેશનમાંથી મેળવેલ નીપજ કઈ છે:
- 3નીચે વિધુતધ્રુવ પોટેન્શિયલ આપેલા છે.View Solution
$Cu^+ /Cu = + 0.52\, V$, $Fe^{3+} /Fe^{2+} = +0.7 7\, V$, $\frac{1}{2}{I_2}\left( s \right)/{I^ - }\, = + 0.54\,V,$ $Ag^+ /Ag = + 0.88\,V$.
ઉપરના પોટેન્શિયલને આધારે, સૌથી પ્રબળ ઓક્સિડેશનકર્તા જણાવો.
- 4જે કોષમાં નીચેની પ્રક્રિયા થતી હોય તેવા કોષનો પ્રમાણિત કોષ પોટેન્શિયલ ($V$ માં) ગણો.View Solution
$F{e^2}+ \left( {aq} \right) + A{g^ + }\left( {aq} \right) \to F{e^{3 + }}\left( {aq} \right) + Ag\left( s \right)$
$E_{Ag^+/Ag}^o = xV$, $E_{F{e^{2 + }}/Fe}^o = yV$, $E_{F{e^{3 + }}/Fe}^o = zV$
- 5પ્રમાણિત ઇલેક્ટ્રોન પોટેન્શિયલ આપેલ છે.View Solution
$F{e^{ + 2 }} + 2{e^ - }\, \to \,Fe\,;\,\,\,\,{E^o} = - 0.440\,V$
$F{e^{ + 3 }} + 3{e^ - }\, \to \,Fe\,;\,\,\,\,{E^o} = - 0.036\,V$
તો $F{e^{ + 3 }} + {e^ - } \to \,F{e^{ + 2 }}$ માટે પ્રમાણિત ઇલેક્ટ્રોન પોટેન્શિયલ $({E^o})$ .............. $\mathrm{V}$ છે.
- 6$298 \mathrm{~K}$. પર કોષ (image) ના વડે તેને વધારી શકાય છે.View Solution
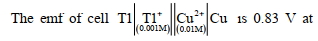
- 7પિગ્લીન ટીન ક્ષારમાંથી $5$ કલાક માટે $2$ એમ્પિયર પ્રવાહ પસાર કરતા $22.2$ ગ્રામ ટીન જમા થાય છે. ક્ષારમાં ટીનની ઓક્સિડેશન અવસ્થા કેટલી છે? ($Sn$ નો પ.ભા. $= 118.69$)View Solution
- 8બટન કોષ $A g-Z n$ માટે $Z n(s)+A g_{2} O(s) \operatorname{Zn} O(s)+2 A g(s)$ ચોખ્ખી પ્રક્રિયા છેView Solution
$\Delta G_{f}^{o}\left(A g_{2} O\right)=-11.21\, kJ\,mol ^{-1}$
$\Delta G_{f}^{o}(Z n O)=-318.3\, kJ \,mol ^{-1}$
ત્યારે $E^{o}$કોષ નો બટન શેલ.........$V$ શું હશે ?
- 9$E^{0} _{Fe^{2+}| Fe}$ $= - 0.441\, V$ અને $E_{F{e^{3 + }}|F{e^{2 + }}}^0\, = \,0.771\,\,V$ હોય, તો $Fe + 2Fe^{3+} \rightarrow 3Fe^{2+}$ પ્રક્રિયાનો પ્રમાણિત $EMF$ .............. $\mathrm{V}$ જણાવો.View Solution
- 10$1 \times 10^{-5}\,M \textrm {AgNO } _ { 3 }$ ને $AgBr$ના $1\,L$ સંતૃપ્ત દ્રાવણમાં ઉમેરવામાં આવે છે.$298\,K$ આ દ્રાવણની વાહકતા $.........\times 10^{-8}\,S\,m ^{-1}$ છે.View Solution
$\left[\right.$ આપેલ : $K _{ sp }( AgBr )=4.9 \times 10^{-13}$ at $298 K$
$\lambda_{ Ag ^{+}}^0=6 \times 10^{-3} Sm ^2\,mol ^{-1}$
$\lambda_{ Br ^{-}}^0=8 \times 10^{-3} Sm ^2\,mol ^{-1}$
$\left.\lambda_{ NO _3^{-}}^0=7 \times 10^{-3} Sm ^2\,mol ^{-1}\right]$
