ચાર તત્વોની ઈલેક્ટ્રોનીય ગોઠવણી કૌંષ માં આપેલ મુજબ છે $L\,\left( {1{s^2},\,\,2{s^2}2{p^1}} \right);\,\,M\,\left( {1{s^2},\,\,2{s^2}\,2{p^5}} \right)$$Q\,\left( {1{s^2},\,\,2{s^2}\,2{p^6},\,\,3{s^1}} \right);\,\,R\,\left( {1{s^2},\,\,2{s^2}\,2{p^2}} \right)$ તે તત્વ કે જે દ્રીપરમાણ્વીય અણુ બનાવે છે
Medium
b
(b) Non-metals readily form diatomic molecules by sharing of electrons. Element \(M\,(1{s^2}\,2{s^2}\,2{p^5})\) has seven electrons in its valence shell and thus needs one more electron to complete its octet. Therefore, two atoms share one electron each to form a diatomic molecule \(({M_2})\)
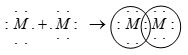
(b) Non-metals readily form diatomic molecules by sharing of electrons. Element \(M\,(1{s^2}\,2{s^2}\,2{p^5})\) has seven electrons in its valence shell and thus needs one more electron to complete its octet. Therefore, two atoms share one electron each to form a diatomic molecule \(({M_2})\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1આવર્ત કોષ્ટકના સમૂહ$-15$માં $NH_3$ $(106^o )$ થી $SbH_3 \,\,(101^o )$ બંધકોણના ઘટતા મૂલ્યોનું કારણView Solution
- 2નીચે આપેલા પરિબળો ની સંખ્યા કે જે આયનિક બંધના ટકાવાર સહસંયોજક પ્રકૃતિ પર આસર કરે છે.તે $.......$ છે.View Solution
$A$.ધન આયન ની ધ્રુવીકરણ શક્તિ (સામર્થ્ય)
$B$.ઋણ આયન ના વિચ્છેદ (વિકૃતિ) ની હદ (વિસ્તાર)
$C$.ઋણ આયન ની ધ્રુવીયતા
$D$. આયન ની ધ્રુવીકરણ શક્તિ (સામર્થ્ય)
- 3View Solutionસંહ-સંયોજક બંધ નિર્માણ પામે છે જ્યારે કોઈ અણુમાં પરમાણુ પાસે ..... હોય છે?
- 4નીચેના સંયોજનો પૈકી કયું સંયોજન એવું છે કે જે ધ્રુવીય છે અને તેમાં કેન્દ્રિય અણુનું સંકરણ $s{p^2} - $ છેView Solution
- 5$NO_2^ + ,NO_3^ - $ અને $NH_4^ + $ માં નાઇટ્રોજનની પરમાણ્વીય કક્ષકોનું સંકરણ નીચેનામાંથી અનુક્રમે ક્યું થયું હશે?View Solution
- 6View Solutionનીચેનામાંથી કોણ કાયમી દ્વિધ્રુવ ચાકમાત્રા ધરાવતું નથી ?
- 7View Solutionઉત્કલન બિંદુનો ખોટો ક્રમ કયો છે?
- 8${CO}$ અને ${NO}$ના બંધ-ક્રમાંક વચ્ચેનો તફાવત ${NO}^{\oplus}$ એ $\frac{{x}}{2}$છે, જ્યાં ${x}=.....$View Solution
- 9View Solutionનીચેનામાંથી કોની દ્વિધ્રુવ ચાકમાત્રા શૂન્ય છે ?
- 10સલ્ફર ટ્રાયોક્સાઇડના ટ્રાઇમરમાં કેટલા $S - S$ બંધ, $S - O - S$ બંધ, $\sigma-$ બંધ, $\pi -$ બંધ હાજર છે?View Solution
