$I.$ ઓક્સિજનનો એક અણુ
$II.$ નાઇટ્રોજનનો એક અણુ
$III.$ $1\times10^{-10}$ મોલ ઓક્સિજન
$IV.$ $1\times10^{-10}$ મોલ કોપર
Mass of $6.023 \times 10^{23}$ atoms of oxygen $= 16\,g$
Mass of one atoms of oxygen $ = \frac{{16}}{{6.023 \times {{10}^{23}}}} = 2.66 \times {10^{ - 23}}\,g$
Mass of $6.023 \times 10^{23}$ atoms of nitrogen $= 14\,g$
Mass of one atom of nitrogen
$ = \frac{{14}}{{6.023 \times {{10}^{23}}}} = 2.32 \times {10^{ - 23}}\,g$
Mass of $1 \times 10^{10}$ mole of oxygen $= 16 \times 10^{-10}$
Mass of $1$ mole of copper $= 63\,g$
Mass of $1$ mole of oxygen $= 16\,g$
Mass of $1 \times 10^{-10}$ mole of copper
$= 63 \times 1 \times 10^{-10}$
$= 63 \times 10^{-10}$
So, the order of increasing mass is
$II < I < III < IV$
Download our appand get started for free
Similar Questions
- 1નીચે આપેલ બંધારણ સાથે $RNA$ માં હાજર યુરેસીલ બેઇઝ છે. યુરિસિલમાં $N$ ના $\%............$ છે.View Solution
મોલર દળ $N =14\,g\,mol ^{-1} ; O =16\,g\,mol ^{-1} ; C =12\,g\,mol ^{-1} ; H =1\,g\,mol ^{-1}$;
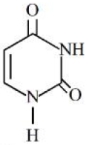
- 2પ્રક્રિયા $2Al{(s)}+6HCl{(aq)} \rightarrow 2Al^{^{3+}}{(aq)}+6Cl^-{(aq)} +3H_2{(g)}$ માં .........View Solution
- 3$0.15\, \mathop A\limits^o$ $ =........ nm$View Solution
- 4$68\, ^oF = ........ K.$View Solution
- 5જ્યાંરે $0.01\, M\, KMnO _{4}$ દ્રાવણ ને $20.0 \,mL\,\, 0.05\, M$ મ્હોર દ્રાવણમાં બ્યુરેટ દ્વારા ઉમેરવામાં આવ્યું. $50\, mL$ બ્યુરેટ નું પ્રારંભિક અવલોકન શૂન્ય છે. અંતિમ બિંદુ બાદ બ્યુરેટમાં બાકી રહેલા $KMnO _{4}$ નું ક્દ......... $mL$ (નજીકના પૂર્ણાકમાં)View Solution
- 6$C_xH_yO_z$ અણુસૂત્ર ધરાવતા $1\, mol$ સંયોજનનુ $O_2$ ની હાજરીમાં સંપૂર્ણ દહન કરવા માટે .... મોલ ઓક્સિજનની જરૂર પડે.View Solution
- 7$500\, g$ દ્રાવણમાં કેટલા ગ્રામ $NaOH$ ઓગાળતા 1$0 \% \,w/w$ સાંદ્રતા ધરાવતું દ્રાવણ બને ?View Solution
- 8જો $200\,mg$ $CO_2$ માંથી $10^{21}$ અણુઓ દુર કરવામાં આવે તો $CO_2$ ના બાકી રહેલા મોલની સંખ્યા ..... હશે.View Solution
- 9ચોર પાસેથી $19.7$ કિ.ગ્રા. સોનું જપ્ત કરવામાં આવ્યું તો તેની પાસેથી સોનાના કેટલા પરમાણુઓ જપ્ત કરવામાં આવેલા હશે? ($Au$ $= 197$ ગ્રામ/મોલ)View Solution
- 10$STP$ પર $O _2$ ના $2.8375\,liters$ માં અણુઓ અને $moles$ ની સંખ્યા અનુક્રમે શોધો.View Solution
