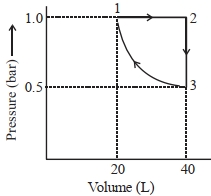
એક મોલ આદર્શ એકપરમાણ્વીય વાયુને આલેખમાં દર્શાવ્યા પ્રમાણે ફેરફાર કરેલ છે. થયેલ કાર્યની માત્રા (પ્રણાલી વડે અથવા પ્રણાલી ઉપર) $..........J$ છે. (નજીકનો પૂર્ણાક) Given : $\log 2=0.3, \ln 10=2.3$

JEE MAIN 2023, Medium
a
$1 \rightarrow 2 \Rightarrow \text { Isobaric process }$
$1 \rightarrow 2 \Rightarrow \text { Isobaric process }$
$2 \rightarrow 3 \Rightarrow \text { Isochoric process }$
$3 \rightarrow 1 \Rightarrow \text { Isothermal process }$
$W = W _{1 \rightarrow 2}+ W _{2 \rightarrow 3}+ W _{3 \rightarrow 1}$
$=\left(- P \left( V _2- V _1\right)+0\left[- P _1 V _1 \ln \left(\frac{ V _2}{ V _1}\right)\right]\right)$
$=\left[-1 \times(40-20)+0+\left[-1 \times 20 \ln \left(\frac{20}{40}\right)\right]\right]$
$=-20+20 \ln 2$
$=-20+20 \times 2.3 \times 0.3$
$=-6.2 \text { bar } L$
$| W |=6.2 \text { bar } 1=620\,J$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1જયારે $60\,W$ ઈલેકિટ્રક હીટર ને વાયુમાં $100\,s$ માટે સમોષ્મી દિવાલો સાથે સમોષ્મી સાથે ના અચળ કદ ના પાત્રમાં $100\,s$માટે ડુબાડવામાં આવે છે.ત્યારે વાયુ નું તાપમાન $5^{\circ}\,C$ વધે છે.આપેલ વાયુ ની ઉષ્માક્ષમતા $........\,J\,k ^{-1}$ છે.(નજીકનો પૂર્ણાક)View Solution
- 2અચળ તાપમાને પ્રતિવર્તી રીતે $0.3344\,J$ ઉષ્મા ઉમેરતા કોઇ એક ચોકકસ પદાર્થના નમૂનાની એન્ટ્રોપી $0.836\,J\,K^{-1}$ જેટલી વધે છે, તો નમૂનાનું તાપમાન .....$K$View Solution
- 3View Solutionસમતાપીય પરિસ્થિતી હેઠળ, એક આદર્શ વાયુના અપ્રતિવર્તિય વિસ્તરણ (irreversible expansion) માટે, સાચો વિકલ્પ શું છે ?
- 4જો $ Fe_2O_3$$_{(s)} + 3CO$ $_{(g)}$ $= 2Fe $$_{(s)} +$$3CO_2$$_{(g)}$ પ્રક્રિયાની પ્રમાણિત ઉષ્મા $ -6.6$ $kcal$ તો $Fe_2O_3$ $_{(s)}$ માટે $\Delta$$H_f$$^o$ $= ......$ $kcal/mol$ [ $\Delta$$H_f$$^o$ $CO$ $_{(g)} = -26.4\, kcal$ અને $\Delta$$H_f$$^o$ $CO_2$ $_{(g)}$$ = -94\, kcal $ ]View Solution
- 5જો $298 \,K$ એ $C-H, C-C, C-C$ અને $H-H$ બંધની બંધ ઉર્જા અનુક્રમે $414, 347, 615$ અને $435 \,kJ $ મોલ $^{-1}$ છે, તો પ્રક્રિયા માટે એન્થાલ્પી ફેરફારનું મૂલ્ય કેટલા .....$kJ$ થશે ? $H_2C = CH_{2(g)} + H_{2(g)} \rightarrow H_3C-CH_{3(g)}$ એ $298\, K$View Solution
- 6જો ઇથેન, હાઇડ્રોજન અને ગ્રેફાઇટની દહન ઉષ્મા અનુક્રમે $-1560$, $-393.5$ અને $-286 \;\mathrm{kJ} / \mathrm{mol},$ હોય, તો ઇથેનની પ્રમાણિત સર્જન ઉષ્મા $\left(\Delta_{t} \mathrm{H}_{298}^{0}\right)$ .......... $kJ/mol$ જણાવો.View Solution
- 7$25\,^oC\,\, 50$ ગ્રામ આયર્નને $HCl $ માં દ્રાવ્ય કરવામાં આવે તો બંધ પાત્રમાં થતાં કાર્યની ગણતરી ..... થશે. વાતાવરણનું દબાણ એક વાતાવરણ છે.View Solution
- 8View Solutionએક બંધ પ્રણાલીમાં દ્વિઆણ્વિય આદર્શ વાયુ માટે નીચે આપેલા આલેખો પૈકી કયો ઊષ્માગતિશાસ્ત્રના વિવિધ પરિમાણો વચ્ચેનો સાચો સંબંધ વર્ણવતો નથી?
- 9જો પ્રક્રિયા માટે $\Delta G^{o} >0$ તો......View Solution
- 10વાયુમય પ્રક્રિયા માટે $A_{(g)} + 3B_{(g)} \rightarrow 3C_{(g)} + 3D_{(g)}$ $27\,^oC$ એ $\Delta U=17 \,Kcal$ છે. ધારો કે $R = 2 \,cal \,K$$^{-1}$ મોલ$^{-1}$ છે તો ઉપરની પ્રક્રિયા માટે $\Delta H$ નું મુલ્ય .......$Kcal$ થશે.View Solution
