એક $p-n$ ફોટો-ડાયોડને $2.0\; eV $ બેન્ડગેપ પદાર્થમાંથી બનાવેલ છે. આ પદાર્થ ઓછામાં ઓછી કઇ આવૃતિવાળા વિકિરણનું શોષણ કરી શકે?
AIPMT 2008, Medium
d
Band gap \(=2\,eV\).
Band gap \(=2\,eV\).
Wavelength of radiation corresponding to this energy,
\(\lambda=\frac{h c}{E}=\frac{12400 \mathrm{eVA}}{2 \mathrm{eV}}=6200 \mathrm{A}\)
The frequency of this radiation
\(=\frac{c}{\lambda}=\frac{3 \times 10^{8} \mathrm{m} / \mathrm{s}}{6200 \times 10^{-10} \mathrm{m} / \mathrm{s}}\)
\(\Rightarrow v=5 \times 10^{14} \mathrm{Hz}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
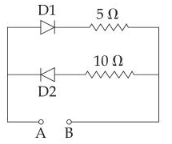
- 1પરિપથમાં દર્શાવેલ $AB$ ને $2\,V$ ની બેટરી સાથે જોડેલ છે.એક કિસ્સામાં જ્યારે બેટરીનો ધન ધ્રુવ $A$ સાથે અને બીજા કિસ્સામાં જ્યારે બેટરીનો ધન ધ્રુવ $B$ સાથે જોડેલો હોય ત્યારે તેમાંથી પસાર થતો પ્રવાહ અનુક્રમે કેટલો હશે?View Solution
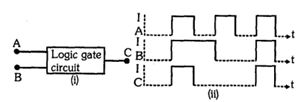
- 2નીચેની આકૃતિમાં $A$ અને $B$ એ ઈનપુટ્સ અને $C$ આઉટપુટ દર્શાવતો લોજીક ગેટ પરિપથ છે. $A, B$ અને $ C $ ના કોલ્ટેજ તરંગ સ્વરૂપો બીજી આકૃતિમાં નીચે દર્શાવ્યા મુજબ છે, તો પરિપથમાં લોજીક ગેટ કયો હશે?View Solution
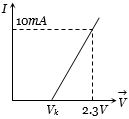
- 3આપેલ ગ્રાફ પરથી જર્મેનિયમ ડાયોડનો અવરોધ કેટલા ......$k\Omega$ થાય? $ ({V_k} = 0.3V) $View Solution
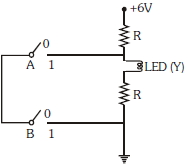
- 4View Solutionઆપેલ સર્કિટ ડાયેગ્રામ વડે રજુ થતું સાચું બુલીયન ઓપરેશન કયું છે
- 5$5 \times 10^{18}\, m^{-3}$ ઇલેક્ટ્રોનની સંખ્યા, $5 \times 10^{19}\, m^{-3}$ હોલની સંખ્યા, $2 .0\, m^2\, v^{-1}\, s^{-1}$ ઇલેક્ટ્રોન મોબિલિટી અને $0.01\, m^2\, v^{ -1}\, s^{-1}$ હૉલ મોબિલિટી ધરાવતા અર્ધવાહક પદાર્થની વાહકતા(${\left( {\Omega - m} \right)^{ - 1}}$ માં) કેટલી થાય? (ઇલેક્ટ્રોનનો વિજભાર$=1.6 \times 10^{-19}\, C$)View Solution
- 6View Solutionનીચેનામાંનો ક્યો પરિપથ રીવર્સ બાયસ થયેલ છે ?
- 7View Solutionશુધ્ધ અર્ધવાહકમાં ઓરડાના તાપમાને ઇલેકટ્રોન અને હોલની સંખ્યા કેટલી હોય?
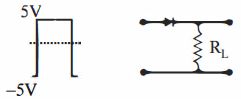
- 8આકૃતિમાં દર્શાવ્યા મુજબ $10 \;V$ નું એક ચોરસ ઈનપુટ સિગ્નલ $P - N$ જંક્શન ડાયોડને આપવામાં આવે છે. તો લોડ અવરોધ $R_{L}$ આગળ આઉટપુટ સિગ્નલ કેવું હશે ?View Solution
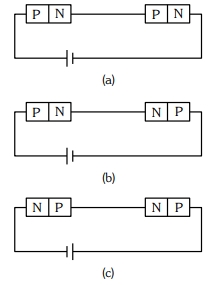
- 9આપેલ પરિપથો $(a), (b)$ અને $(c$) માં, $p-n$ જંક્શનને સમાંતર સ્થિતિંમાનનો તફાવત$.....$માં સમાન છે.View Solution
- 10View Solutionનીચે આપેલ ડાયોડ પરિપથમાંથી કયો ડાયોડ ફોરવર્ડ બાયસમાં છે?
