હાઇડ્રોજન અણુ સ્પેક્ટ્રમમાં કયા સંક્રમણમાં સંક્રમણની સમાન તરંગલંબાઇ હશે,$He^+$નું સ્પેક્ટ્રમ$n = 4$ to $n = 2$?
AIIMS 2016, Medium
d
For \(He^+\) ion, \(\frac{1}{\lambda } = {Z^2}R\left[ {\frac{1}{{n_1^2}} - \frac{1}{{n_2^2}}} \right]\)
For \(He^+\) ion, \(\frac{1}{\lambda } = {Z^2}R\left[ {\frac{1}{{n_1^2}} - \frac{1}{{n_2^2}}} \right]\)
\({(2)^2}R\left[ {\frac{1}{{{2^2}}} - \frac{1}{{{4^2}}}} \right] = \frac{{3R}}{4}\)
For hydrogen atoms, \(\frac{1}{\lambda } = R\left[ {\frac{1}{{n_1^2}} - \frac{1}{{n_2^2}}} \right]\)
\(\frac{{3R}}{4} = R\left[ {\frac{1}{{n_1^2}} - \frac{1}{{n_2^2}}} \right]\,\frac{1}{{n_1^2}} - \frac{1}{{n_2^2}} = \frac{3}{4}\)
\(n_1 = 1\) and \(n_2 = 2.\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1વિકિરણ કે જેની આવૃતિ $5 \times 10^{10} \,s^{-1}$ છે ત્યારે એક મોલ ફોટોનની ઊર્જા …....... $\mathrm{J}$View Solution
- 2$Cl_2$ માં રહેલા એક મોલ $Cl-Cl$ બંધ તોડવા જરૂરી ઊર્જા $242\, kj \, mol^{-1}$ છે. તો એક $Cl-Cl$ બંધ તોડવા જરૂરી પ્રકાશની મહતમ તરંગલંબાઈ .......... $\mathrm{nm}$ થશે.View Solution
$(C= 3 \times 10^8 \,ms^{-1},N_A = 6.02 \times 10^{23} mol^{-1}).$
- 3કઈ કક્ષકને $\Psi 310$ તરંગ વિધેય દ્વારા દર્શાવવામાં આવે છે ?View Solution
- 4$x$ અને $y$ બે ગતિશીલ કણો છે. $x$ ના વેગમાનના માપનની અચોક્કસતા $y$ ના વેગમાનની અચોક્કસતા કરતાં અડધી છે. જો કણ સ્થાનની અનિશ્વિતતા $ 0.05 Å$ હોય, તો $y$ કણના માપનમાં રહેલી સ્થાનની અનિશ્વિતા = .......View Solution
- 5View Solutionછઠ્ઠી હરોળમાં, કક્ષા કઈ રીતે ભરાઈ છે?
- 6$H$ પરમાણુની આયનીકરણ ઊર્જા $13.6 \,eV$ છે. તો ભૂમિ અવસ્થામાંથી ઊંચી અવસ્થામાં જોવા મલે છે. ઉત્તેજીત થવા માટે જરૂરી ઊર્જા ... $eV$ થશે.View Solution
- 7View Solutionનીચેનામાંથી કયો નિયમ વિવિધ કક્ષામાં ઈલેકટ્રોન ભરાવાનો ક્રમ સૂચવે છે?
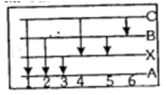
- 8આક્રૂતિ દર્શાવે છે કે ઉત્સર્જીત વર્ણપટ્ટમાં છ વર્ણપટ્ટ રેખાઓના ઊર્જા સ્તર ઉદભવે છે. (ઉ.દા. $B$ થી $X$ તરફ થતું સંક્રમણમાંથી $5$ રેખા ઉદભવે છે.) જે નીચેનામાંથી કઈ વર્ણપટ્ટ રેખા અવશોષણ વર્ણપટ્ટ ધરાવતો નથી.View Solution
- 9$CH_4$ અને $O_2$ અણુઓના વેગનો ગુણોત્તર શું હોવો જોઈએ કે જેથી તેને સમાન તરંગલંબાઈ વાળા દ-બ્રોગ્લી તરંગ સાથે સાંકળી શકાય તેની સાથે જોડાયેલ દ બ્રોગ્લી તરંગના સમાન તરંગની લંબાઈમાં કેટલો હશે ?View Solution
- 10કઈ કક્ષકને $\Psi 310$ તરંગ વિધેય દ્વારા દર્શાવવામાં આવે છે ?View Solution
