હાઈડ્રોજન વાયુને ખૂબ જ ઉંચા તાપમાને ગરમ કરવામાં આવે છે, ત્યારે તેના અણુઓની ચાકગતિની ઊર્જા અને કુલ ઊર્જાનો ગુણોત્તર ક્યા અપુર્ણાકથી મળશે.
Medium
b
(b)
(b)
Hydrogen is a diatomic molecules and if vibrational degrees of freedom are increased the degrees of freedom will be \(3\) translation \(2\) rotational and two vibrational.
\(\therefore\) So total \(7\) degree of freedom.
Fraction of energy possessed due to rotational motion : Degree of freedom due to rotation total degree of freedom \(=\frac{2}{7}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$30$ લિટર કદના સિલિન્ડરમાં ઓક્સિજનનું પ્રારંભિક ગેજ દબાણ $15\, atm$ અને તાપમાન $27° C$ છે. સિલિન્ડરમાંથી અમુક ઓક્સિજન બહાર નીકળી જાય છે ત્યારે ગેજદબાણ $11\, atm$ અને તાપમાન $17°C$ ઘટી જાય છે. સિલિન્ડરમાંથી બહાર આવેલા ઓક્સિજનનું દળ ........ $kg$ થશે. $R = 8.31 \,J mol^{-1} K^{-1},\, O_2 = 32\, u.$View Solution
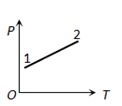
- 2$P \rightarrow T$ આલેખ એ વાયુને ગરમ કરતા મળે છે. આ પ્રક્રિયા દરમિયાન બિંદુ $1$ થી $2$ કદ......View Solution
- 3જ્યારે $Q$ ઉષ્મા આપવામાં આવે ત્યારે દઢ એક પરમાણ્વિક વાયુ $Q / 5$ જેટલું કાર્ય કરે છે. આ રૂપાંતરણ દરમ્યાન વાયુની મોલર ઉષ્માધરીતા $\frac{ x R }{8}$ છે. $x$ નું મૂલ્ય ...... છે. $[R =$ વાયુ નિયતાંક $]$View Solution
- 4વાયુનું તાપમાન $T$ અને કદ $V$ છે.અચળ દબાણે તાપમાન $ \Delta T, $ વધારતાં કદમાં $ \Delta V $ વધારો થાય છે,તો $ \delta = \Delta V/(V\Delta T) $ નો તાપમાન સાથે ફેરફાર દર્શાવતો આલેખ નીચે પૈકી ક્યો છે?View Solution
- 5View Solutionઅચળ કદ માટે એક પરમાણ્વીય વાયુનો ઉષ્મા નિયતાંકનો આલેખ કયો છે?
- 6એક બંધ પાત્રમાં ભરેલા વાયુને $1{ }^{\circ} C$ તાપમાને ગરમ કરવામાં આવે છે ત્યારે તેનું દબાણ $0.4 \%$ જેટલું વધે છે. વાયુનું પ્રારંભિક તાપમાન ..........$K$ હશે.View Solution
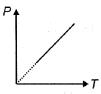
- 7એક પરમાણ્વિક આદર્શ વાયુનું દબાણ $P$ તેના નિરપેક્ષ તાપમાન $T$ સાથે આકૃતિમાં દર્શાવ્યા મુજબ બદલાય છે. આ પ્રક્રિયા દરમિયાન વાયુની મોલાર ઉષ્મા ક્ષમતા ....... $R$ છે? [$R$ એ વાયુ અચળાંક છે.]View Solution
- 8$30$ લિટર કદના સિલિન્ડરમાં ઓક્સિજનનું પ્રારંભિક ગેજ દબાણ $15\, atm$ અને તાપમાન $27° C$ છે. સિલિન્ડરમાંથી અમુક ઓક્સિજન બહાર નીકળી જાય છે ત્યારે ગેજદબાણ $11\, atm$ અને તાપમાન $17°C$ ઘટી જાય છે. સિલિન્ડરમાંથી બહાર આવેલા ઓક્સિજનનું દળ ........ $kg$ થશે. $R = 8.31 \,J mol^{-1} K^{-1},\, O_2 = 32\, u.$View Solution
- 9${N}_{2}$ વાયુના અણુંની કયા તાપમાને ગતિઊર્જા $0.1\;volt$ થી પ્રવેગિત કરેલા સ્થિર ઇલેક્ટ્રોનની ગતિઊર્જા જેટલી થાય? (નજીકના પૂર્ણાંકમાં)View Solution
$\left(\right.\left.{k}_{{B}}=1.38 \times 10^{-23} \, {J} / {K}\right)$
- 10$ 0^\circ C $ તાપમાને બેરોમીટરનું દબાણ $760\, mm$ છે.તો $ 100^\circ C $ તાપમાને બેરોમીટરનું દબાણ કેટલું થાય?View Solution
