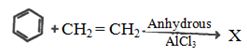Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીપજ $(A)$ શું છે ?View Solution
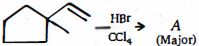
- 2${{H}_{2}}\overset{14}{\mathop{C}}\,=CH-C{{H}_{3}}\xrightarrow[ or\,high\text{ }temp]{low\text{ }conc \text{ }of\text{ }B{{r}_{2}}}(?)$View Solution
ઉપરોક્ત પ્રકિયા ની નીપજ શું હશે ?
- 3ની પ્રક્રિયા $HBr$ સાથે.......આપે છે.View Solution
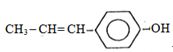
- 4પ્રોપીનની પ્રક્રિયા $HOCl$ સાથે કરાવતા યોગ ક્યા માર્ગ દ્વારા આગળ વધે છે?View Solution
- 5નીચે આપેલી પ્રક્રિયામાં $C{H_3} - C{H_2} - C{H_2} - C{H_3} \xrightarrow[475\, K]{H_2SO_4}$View Solution
- 6View Solutionજંતુનાશક ગેમેક્જિન = .......
- 7$\begin{matrix}View Solution
Cl \\
| \\
Ph-C-C{{H}_{3}} \\
| \\
Cl \\
\end{matrix}\xrightarrow{3NaN{{H}_{2}}}\underset{\Pr oduct}{\mathop{(A)}}\,;$નીપજ $(A)$ શું હશે ? - 8કયા પ્રક્રિયક દ્વારા $1$-બ્યુટાઈન અને $2$-બ્યુટાઈન વચ્ચે ભેદ પારખી શકાય છે ?View Solution
- 9નીપજ $(Z)$ શું હશે ?View Solution
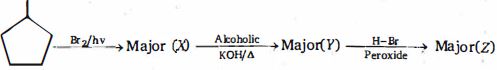
- 10પ્રક્રિયામાં $'X' $ શું છે ?View Solution