$\frac{1}{2}C{l_2}(g)\xrightarrow{{\frac{1}{2}{\Delta _{diss}}{H^\Theta }}}Cl(g)\xrightarrow{{{\Delta _{eg}}{H^\Theta }}}$ $C{l^ - }(g)\xrightarrow{{{\Delta _{Hyd}}{H^\Theta }}}C{l^ - }(aq)$
તો $\frac{1}{2}C{l_2}(g)$ ના $Cl^-_{(aq)}$ માં રૂપાંતમાં ઊર્જાનો ફેરફાર ............. $\mathrm{kJ\,mol}^{-1}$ જણાવો.
$({{\Delta _{diss}}H_{C{l_2}}^\Theta } = 240\,kJ\,mol^{-1}, {{\Delta _{eg}}H_{C{l}}^\Theta }= -349 \,kJ\,mol^{-1},$${{\Delta _{Hyd}}H_{C{l}}^\Theta }= -381 \,kJ\,mol^{-1})$
The energy involved in the conversion of
\(\frac{1}{2} C_{2}(g)\) to \(C l^{-1}(a q)\) is given by
\(\Delta H=\frac{1}{2} \Delta_{d i s s} H_{C l_{2}}^{(-)}+\Delta_{e g} H_{C l}^{(-)}+\Delta_{h y l} H_{C l}^{(-)}\)
Substituting various values from given data, we get
\(\Delta H=\left(\frac{1}{2} \times 240\right)+(-349)+(-381)\, k J \,m o l^{-1}\)
\(=(120-349-381)\, k J\, m o l^{-1}\)
\(=-610 \,k J \,m o l^{-1}\)
Download our appand get started for free
Similar Questions
- 1$CO ({-1}10 \,KJ\, mol^{-1}$) અને $CO_2(-394 \,KJ$ મોલ$^{-1}$) ના નિર્માણની પ્રમાણિત એન્થાલ્પી છે. જ્યારે $1$ મોલ ગ્રેફાઈટ સળગે ત્યારે દહન ઉષ્મા કેટલા ............... $\mathrm{KJ}$ થશે ?View Solution
- 2View Solutionનીચેના પૈકી ક્યુ સાચું છે ?
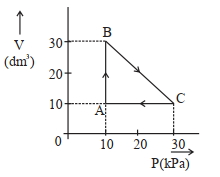
- 3$Image$ એક આદર્શ વાયુ બિંદુ $A$ થી શરૂઆત કરીને ચક્રિય સ્થાનાંતર કરે છે અને ઉપરની આકૃતિમાં દર્શાવ્યા પ્રમાણે દોરેલા પથ $\mathrm{A} \rightarrow \mathrm{B} \rightarrow \mathrm{C} \rightarrow \mathrm{A}$ દૃવારા તે જ બિંદુ પર પાછો ફરે છે. આ પ્રક્રમમાં થયેલ કુલ કાર્ય ____________$\mathrm{J}$ છે.View Solution
- 4$C + O_2 \rightarrow CO_2$ પ્રક્રિયા માટે એન્થાલ્પી ...... હોય છે.View Solution
- 5નીચેના પ્રકમો પરથી પ્રકિયા $B + D \rightarrow E + 2C$ માટે $\Delta H$ નું મૂલ્ય કેટલા ........... $\mathrm{kJ/mol}$ હશે તે જણાવો.View Solution
$\Delta H \,(kJ/mol)$ $\frac 12 A \rightarrow B$ $+150$ $3B \rightarrow 2C + D$ $-125$ $E + A \rightarrow 2D$ $+350$ - 6View Solutionનીચેનામાંથી કઈ ઉષ્માશોષક પ્રક્રિયા સ્વયંભૂ છે
- 7$C_{(s)} + O_{2(g)} \rightarrow CO_{2(g)}$ માટે ......View Solution
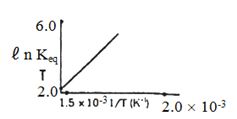
- 8પ્રક્રિયા માટે $ℓ\,n\, K_{eq}$ વિરૂદ્ધ તાપમાનનો આલેખ દોરવામાં આવે તો પ્રક્રિયા...... હોવી જોઈએ.View Solution
- 9જો કોઈ પદાર્થ માટે ગલનબિંદુ $T_B$ તથા ઠારબિંદુ $T_A$ હોય તો એન્ટ્રોપીમાં થતા ફેરફાર અને તાપમાન વચ્ચેનો સાચો સંબંધ કયા આલેખ વડે દર્શાવી શકાય ?View Solution
- 10$K_c$ $P + Q $ $\rightleftharpoons$ $ R + C$ એ $10^{-2}$ છે અને $K_f $ $=$ $10^{-1}$ છે તો પ્રતિગામી સક્રિય માટે દર અચળાંક ....... થશે.View Solution
