\(9. 65\) ampere current was passed for \(1 .0\) hour ( \(3600\) seconds) Number of moles of electrons passed \( = \frac{{I(A) \times t(s)}}{{96500}} = \frac{{9.65A \times 3600s}}{{96500}} = 0.36\,moles\)
\({C_6}{H_5}N{O_2}\, + \,4{e^ - } + 4{H^ + } \to \,{\text{p - Aminophenol}} + {H_2}O\)
\(4\) moles of electrons will reduce \(1\) mole of nitrobenzene to \(p-\) aminophenol. \(0.36\) moles of electrons will reduce \(\frac{{0.36}}{4} = 0.09\) moles of nitrobenzene to \(p-\) aminophenol \(p-\) aminophenol molar mass \(= 109 .14\,\,g/mol\) Mass of \(p-\) aminophenol obtained \(= 109.14\,g/mol \times 0.09\,mol = 9.81\,g\)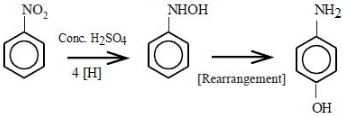
Download our appand get started for free
Similar Questions
- 1નીચે આપેલા અર્ધકોષોથી બનતા કોષનો $EMF$ કેટલા ............ $\mathrm{V}$ થશે?View Solution
$M{g^{2 + }} + 2{e^ - } \to Mg(s);\,\,E = - 2.37\,V$
$C{u^{2 + }} + 2{e^ - } \to Cu(s);\,\,\,E = + 0.33\,V$
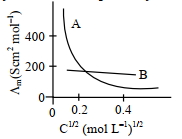
- 2વિધૂતવિભાજયો $A$ અને $B$ માટે મોલર વાહકતાઓ વિરુધ્ધ $C C1/2$ નો ગ્રાફ (આલેખ) નીચે દર્શાવેલ છે. વિધુતવિભાજ્યો $A$અને $B$ અનુક્રમે શોધો :View Solution
- 3કયુ સમીકરણ એ વિદ્યુત વિભાજનનો પ્રથમ નિયમને દર્શાવે છે? ($m \rightarrow$ દળ $c \rightarrow $ પ્રવાહ $t \rightarrow$ સમય)View Solution
- 4નીચેનામાંથી કયો સંબંધ પ્રમાણભૂત ઇલેક્ટ્રોડ સંભવિત અને સંતુલન સ્થિરતા વચ્ચેનો સાચો સંબંધ રજૂ કરે છે ?View Solution
$I$. $\log \,\,K\, = \,\frac{{nF{E^o}}}{{2.303\,RT}}$
$II$. $K\, = \,{e^{\frac{{nF{E^o}}}{{RT}}}}$
$III$. $\log \,\,K\, = -\,\frac{{nF{E^o}}}{{2.303\,RT}}$
$IV$. $\log \,\,K\, = 0.4342\,\,\frac{{-nF{E^o}}}{{RT}}$
સાચું વિધાન $(s)$ પસંદ કરો
- 5આલ્કલી તત્વો $A,\,B,\,C$ અને $D$ નાં પ્રમાણિત વિદ્યુતધ્રુવ પોટૅન્શિયલ $( E^{0}_{Red} )$ અનુક્રમે $-3.05\,V,\,\, -1.66\, V, \,\,-0.40\,V$ અને $0.80\,V$ છે, તો ...... સૌથી વધુ પ્રતિક્રિયાત્મક (ક્રિયાશીલ) હશે ?View Solution
- 6આપેલ - $E_{F{e^{ + 3}}/Fe}^ \circ = \,\, - \,0.036\,\,V,\,\,E_{F{e^{ + 2}}/\,Fe}^ \circ \,\, = \,\, - 0.439\,\,V$ પરિવર્તન માટે પ્રમાણિત ધ્રુવ પોટેન્શિયલનું મૂલ્ય કેટલા ................. $\mathrm{V}$ થાય? $Fe^{+3}_{(aq)} + e^{-} \rightarrow Fe^{+2}_{(aq)}$View Solution
- 7View Solutionનીચેનામાંથી કઈ ધાતુ તેના ક્ષારના જલીય દ્રાવણના વિદ્યુતવિભાજય પર મેળવી શકાતી નથી.
- 8કઈ ધાતુઓ બેટરી ઉદ્યોગોમાં ઉપયોગી છે.View Solution
$A$. $\mathrm{Fe}$ $B$. $\mathrm{Mn}$ $C$. $\mathrm{Ni}$ $D$. $\mathrm{Cr}$ $E$. $\mathrm{Cd}$
Choose the correct answer from the options given below:
- 9$25^{\circ} {C}$ એ કોષને અનુસરો.View Solution
${Zn}\left|{Zn}^{2+}({aq}),(1 {M}) \| {Fe}^{3+}({aq}), {Fe}^{2+}({aq})\right| {Pt}({s})$
કોષ પોટેન્શિયલ $1.500\, {~V}$ પર ${Fe}^{3+}$ આયન તરીકે હાજર કુલ આયનનો અપૂર્ણાંક, ${X} \times 10^{-2}$ છે. $X$ નું મૂલ્ય $.....$ (નજીકના પૂર્ણાંકમાં) છે.
$\left(\right.$ આપેલ છે: $\left.E_{{Fe}^{3+} / {Fe}^{2+}}^{0}=0.77\, {~V}, {E}_{{Zn}^{2+} / {Zn}}^{0}=-0.76 \,{~V}\right)$
- 10$NiSO_4$ અને $CuSO_4$ ના દ્રાવણ ભરેલા બે કોષને શ્રેણીબદ્ધ કરી વિદ્યુતપ્રવાહ પસાર કરતાં $1.6$ ગ્રામ $Cu$ વિદ્યુતધ્રુવ પર જમા થાય ત્યારે કેટલી$Ni$ ધાતુ ઉદભવે ? જો $NiSO_4$ ને બદલે $AgNO_3$ નું દ્રાવણ ભરેલો કોષ જોડવામાં આવે, તો સિલ્વર કેટલા ગ્રામ મળે ? [પરમાણુભાર :$ Cu = 63.5$ ગ્રામ/મોલ, $Ni = 58.7$ ગ્રામ/મોલ, $Ag = 108$ ગ્રામ/મોલView Solution
