(b)
This cycle is one of the foundations of the second law of thermodynamics, and Carnot is often considered the father of thermodynamics. He was one of the pioneers who first determined an idealistic way of converting heat energy into work done. Carnot cycle is one of the most efficient heat engines.
Carnot cycle consists of the following four processes:
\(I.\) The gas goes through an isothermal expansion at a high temperature. In this process the gas takes \(q_{\text {in }}\) amount of heat from the surrounding and does \(w_1\) amount of work on the surrounding.
\(II.\) The gas then undergoes a reversible adiabatic expansion. Hence, the temperature of the gas comes down to a lower temperature \(T_{\text {low }}\).
\(III.\) Then the gas is compressed isothermally at \(T_{\text {low }}\) temperature. In this process, the gas loses \(q_{\text {out }}\) amount of heat, and surroundings do work on the gas.
\(IV.\) Now the gas goes through a reversible adiabatic compression which makes the temperature rise up to \(T_{\text {high }}\).
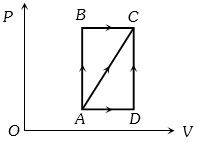
The following diagram shows the \(P-V\) diagram of the Carnot's cycle.
Hence, Carnot's cycle consists of two isothermal and two adiabatic processes.
Download our appand get started for free
Similar Questions
- 1આદર્શ વાયુ માટે આપેલ તાપમાન $T$ માટે $\gamma = \frac{{{C_p}}}{{{C_v}}} = 1.5$ છે.જો વાયુને પોતાના કદથી ચોથા ભાગના કદમાં સ્મોષ્મિ રીતે સંકોચવામાં આવે તો અંતિમ તાપમાન ...... $T$ થાય.View Solution
- 2અચળ દબાણ $100\, N/m^2$ એ વાયુનું કદ $2\,m^3$ થી $1\,m^3$ થાય છે.પછી તેને અચળ કદે ગરમ કરવા $150\, J$ ઉષ્મા આપવામાં આવે છે. વાયુની આંતરિક ઉર્જામાં કેટલો ફેરફાર થાય?View Solution
- 3આકૃતિમાં એક થરમોડાઇનેમિક પ્રક્રિયા દર્શાવેલ છે.આકૃતિમાં દર્શાવેલ બિંદુઓએ દબાણ અને કદ આ મુજબ છે.View Solution
${P_A} = 3 \times {10^4}\;Pa,\;{P_B} = 8 \times {10^4}\;Pa$ અને ${V_A} = 2 \times {10^{ - 3}}\;{m^3},\;{V_D} = 5 \times {10^{ - 3}}\;{m^3}$
$AB$ પ્રક્રિયામાં તંત્રમાં $600\;J$ ઉષ્મા ઉમેરવામાં આવે છે અને $BC$ પ્રક્રિયામાં $200\;J$ ઉષ્મા ઉમેરવામાં આવે છે. $AC$ પ્રક્રિયા દરમિયાન થતો આંતરિક ઊર્જાનો ફેરફાર ..... $J$ હશે.
- 4જો ઠારણ વ્યવસ્થાનું તાપમાન નિરપેક્ષ શૂન્ય પર છે, તો કાર્નોટ એન્જિનની કાર્યક્ષમતા ......... $\%$ થશે.View Solution
- 5$5$ મોલ વાયુનું $500\,K$ અચળ તાપમાને કદ બમણું કરતાં થતું કાર્ય ....... $J$View Solution
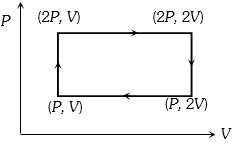
- 6View Solutionનીચે આપેલી ચક્રીય પ્રક્રિયામાં થતું કાર્ય
- 7View Solutionવિધાન : સમોષ્મી વિસ્તરણમાં હમેશા તાપમાન ઘટે
કારણ : સમોષ્મી પ્રક્રિયામાં કદ તાપમાનના વ્યસ્ત પ્રમાણમાં હોય
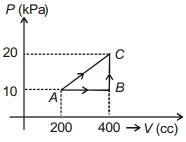
- 8જો વાયુને $A$ થી $C$ સુધી $B$ મારફતે લઈ જવામાં આવે તો વાયુ વડે શોષતી ઉષ્મા $8 \,J$ છે તો તેને $A$ થી $C$ સુધી સીધી રીતે લઈ જવામાં વાયુ વડે શોષાતી ઉષ્મા ............. $J$ છે.View Solution
- 9એક રેફ્રિજરેટરમાં રાખેલ ખોરાકને $9 \,^oC$ તાપમાને સાચવવાનો છે. જો ઓરડાનું તાપમાન $36 \,^oC$ હોય, તો પરફોર્મન્સ-ગુણાંક (કાર્ય સિદ્ધિ ગુણાંક) શોધો.View Solution
- 10$P (V-b)=RT$ સ્થિતિ સમીકરણનું પાલન કરતા એક મોલ વાયુને $(P_1-V_1)$ અવસ્થામાંથી $(P_2-V_2)$ અવસ્થામાં એવી રીતે વિસ્તારિત કરવામાં આવે છે કે જેથી $P-V$ આલેખ સીધી રેખા મળે છે. તો કાર્ય શેના વડે આપવામાં આવે છે?View Solution
