($Cu$ નું આણ્વિય દળ $63\,u$)
$W=Zit$
where $Z\,=$ Electrochemical equivalent
Eq. wt. of copper $=\,\frac {63}{2}=31.5$
$Z\,=\,\frac {31.5}{96500}$
$W\, = \,Zit\, = \,\frac{{31.5}}{{96500}}\, \times \,1.5\, \times 10 \times 60\, = \,0.2938\,g$
Download our appand get started for free
Similar Questions
- 1આપેલ $E^o_{Cl_2/Cl^-} = 1.36\,V,$ $E^o_{Cr^{3+}/Cr}= -0.74\,V,$View Solution
$E^o_{Cr_2/O_7^{2-}/Cr^{3+}}=1.33\,V,$ $E^o_{MnO^-_4/Mn^{2+}} = 1.51\,V$
તો નીચેના પૈકી સૌથી પ્રબળ રિડક્શતકર્તા ..........
- 2View Solutionકઈ પ્રક્રિયાના સમીકરણ દ્વારા ઝીંક નો પ્રમાણિત રીડક્શન પોટેશિયલ દર્શાવી શકાય?
- 3જો દ્રાવણની મોલર વાહકતા $1.26 × 10^{2}\, \Omega^{-1}\, cm^{2}\, mol^{-1}$ અને મોલારિટી $0.01$ હોય, તો તેની વિશિષ્ટ વાહકતા કેટલી થશે ?View Solution
- 4$\frac{1}{2} H _2( g )+ AgCl ( s ) \rightleftharpoons H ^{+}( aq )+ Cl ^{-}( aq )+ Ag ( s )$ પ્રક્રિયા એ આપેલ કયા ગેલ્વોનિક કોષ માં થાય છે ?View Solution
- 5View Solutionનીચેનામાંથી કઈ પ્રક્રિયાનો ઉપયોગ બળતણ કોષ બનાવવા માટે થાય છે
- 6રીડક્શન પોટેન્શિયલનું મૂલ્ય નીચે આપેલ છે. કયો સૌથી શ્રેષ્ઠ રીડ્યુસીંગ કર્તા છે?$Al^{3+}/Al = -1.67 volt, Mg^{2+}/ Mg = -2.34 volt, Cu^{2+}/Cu $View Solution
$= + 0.34 \,volt, I_2/ 2I- = + 0.53\, volt$
- 7$E _{ Cu ^{2+} \mid Cu }^{\circ}=+0.34 V$View Solution
$E _{ Zn ^{2}+\mid Zn }^{ o }=-0.76 V$
ઉપરોક્ત કોષ માટે નીચેના વિકલ્પોમાંથી ખોટા વિધાનની ઓળખ આપો
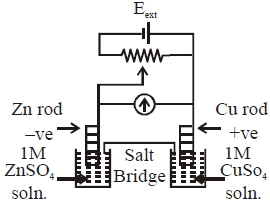
- 8$Cu^{2+}_{(aq)} + e^- \rightarrow Cu^+_{(aq)}$ તથાView Solution
$Cu^+_{(aq)} + e^- \rightarrow Cu_{(s)}$ માટે વિધુતધ્રુવ પોટેન્શિયલ અનુક્રમે $+ 0.15\, V$ તથા $+ 0.50\, V$ છે. $E^o_{Cu^{2+}/Cu}$ ....... $V$ થશે.
- 9$25^{\circ} C$ તાપમાને કોષનો $emf$ ($V$ માં) શોધોView Solution
કોષ રચના: $M \left|\underset{0.01}{ M ^{2+}} \| \underset{0.0001}{ M ^{2+}}\right| M$
(આપેલ છે: $\frac{ RT }{ F }$ in $10=0.06$ ) $E _{ Cell }^{o}$ની કિંમત $4$ $volt$ છે.
- 10$x$ અને $ y$ ધરાવતા દ્રાવણમાંથી વાયુ $z$ ના $(Bubbles)$ ઊભરા આવે છે. જો રિડક્શન પોટેન્શિયલનો ક્રમ $x > y > z$ હોય તો ......View Solution
