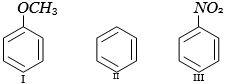
(c) $I > II > III$
Methoxy group is electron releasing it increases electron density of benzene nucleus while $ - N{O_2}$ decreases electron density of benzene.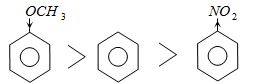
Download our appand get started for free
Similar Questions
- 1$\mathop {Ph - N{H_2},}\limits_{(A)} \,\,\mathop {Ph - NH - Me,}\limits_{(B)} \,\,\mathop {\begin{array}{*{20}{c}}View Solution
{Ph - N - Me}\\
|\\
{Me}
\end{array}}\limits_{(C)} $બેઝિક પ્રબળતા નો ક્રમ કયો છે
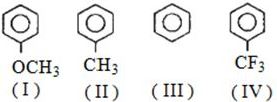
- 2View Solutionનીચેના સમૂહોમાંથી ઓર્થો અને પેરા નિર્દેંશક સમૂહ ...... છે.
- 3આપેલા સંયોજનો $CH \equiv CH,\,C{H_3} - C \equiv CH,\,$ અને $C{H_2} = C{H_2}$ માટે તેમના એસિડ શક્તિનો સાચો ક્રમ નીચેનામાથી કયો થશે?View Solution
- 4View Solutionનીચેના પદાર્થોમાં કેન્દ્રાનુરાગી વિસ્થાપન માટે પ્રક્રિયાશીલતાનો ઘટતો ક્રમ કયો છે ?
- 5એ.....View Solution
$\begin{matrix}
\overset{\Theta }{\mathop{\overset{\centerdot \,\centerdot }{\mathop{C}}\,}}\,{{H}_{2}}-C-C{{H}_{3}} \\
|| \\
O \\
\end{matrix}$ અને $\begin{matrix}
C{{H}_{2}}=C-C{{H}_{3}} \\
| \\
:\underset{\Theta }{\mathop{\underset{\centerdot \,\centerdot }{\mathop{O}}\,}}\,: \\
\end{matrix}$ - 6View Solutionનીચેના અણુઓ માટે સ્થાયીતાનો સાચો ક્રમ (ઘટતો ક્રમ) ગોઠવો.
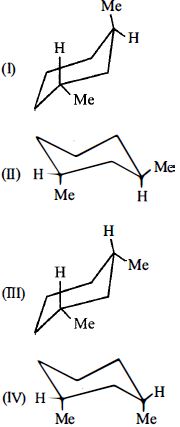
- 7એ.....View Solution
$\begin{matrix}
\overset{\Theta }{\mathop{\overset{\centerdot \,\centerdot }{\mathop{C}}\,}}\,{{H}_{2}}-C-C{{H}_{3}} \\
|| \\
O \\
\end{matrix}$ અને $\begin{matrix}
C{{H}_{2}}=C-C{{H}_{3}} \\
| \\
:\underset{\Theta }{\mathop{\underset{\centerdot \,\centerdot }{\mathop{O}}\,}}\,: \\
\end{matrix}$ - 8વિવિધની એસિડિક પ્રબળતા નો ઘટતો ક્રમ -$(-OH)$ જૂથો કયા છે ?View Solution
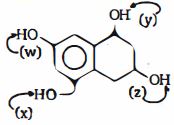
- 9વિભાગ $A$ અને વિભાગ $B$ સંદર્ભેં સાચા જવાબ માટે યોગ્ય વિકલ્પ પસંદ કરો :View Solution
વિભાગ $A$
વિભાગ $B$
$(1)$ મુકત મૂલક(Free radical)
$(A)$ લુઇસ બેઈઝ
$(2)$ ઇલેકટ્રોન અનુરાગી (Electrophile)
$(B)$ વિધુત તટસ્થ
$(3) $કેન્દ્રઅનુરાગી (Nucleophile)
$(C)$ સંયોજકતા કક્ષામાં ઇલેકટ્રોન અષ્ટક ઍસિડ
$(D)$ લુઇસ ઍસિડ
$(E)$ ઇલેકટ્રોન અષ્ટક અપૂર્ણ અને સંયોજકતા કક્ષામાં એકી સંખ્યાના ઇલેક્ટ્રોનો
$(F)$ ઇલેકટ્રોન અષ્ટક અપૂર્ણ
- 10View Solutionનીચેના અણુઓમાં કાર્બન-કાર્બન બંધ લંબાઈનો ક્રમ કયો છે ?
