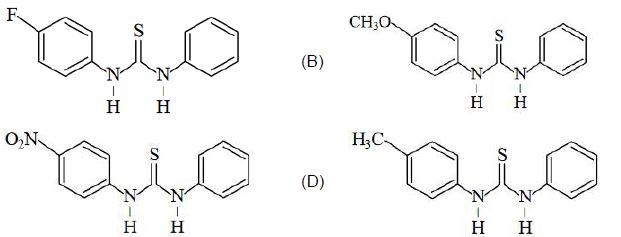
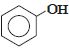
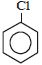
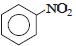
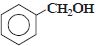
નીચેનામાંથી કયું ઇલેક્ટ્રોન અનુરાગી હુમલા તરફ સૌથી વધુ પ્રતિક્રિયાશીલ છે?
AIPMT 2008, Medium
a
The group showing electron-donating effect (such as \(-NH _{2},- OH)\) should stabilise the intermediate ions, i.e. makes the ring more reactive towards electrophilic substitution than benzene and are called activating group while electron withdrawing group (such as \(\left.-C l, N O_{2}\right)\) increases the positive charge on ring, thus deactivates the ring. Hence, phenol is more readily attacked by an electrophile
The group showing electron-donating effect (such as \(-NH _{2},- OH)\) should stabilise the intermediate ions, i.e. makes the ring more reactive towards electrophilic substitution than benzene and are called activating group while electron withdrawing group (such as \(\left.-C l, N O_{2}\right)\) increases the positive charge on ring, thus deactivates the ring. Hence, phenol is more readily attacked by an electrophile
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
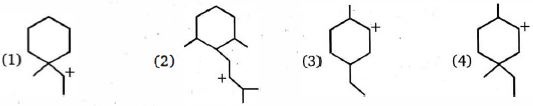
- 1View Solutionનીચેનામાંથી કયો કાર્બન આયન સૌથી ઓછો સ્થિર છે ?
- 2View Solutionનીચેનામાંથી કઇ નીપજ ગોઠવણ કરશે?
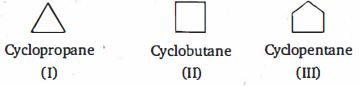
- 3View Solutionનીચેનામાંથી કયા સંયોજનોમાં સૌથી વધુ એસિડિક હાઇડ્રોજન છે?
- 4$C{H_3}C{H_2}OH$ના કયા બંધમાં વિષમાંગ જોડાણ સૌથી સહેલાઇથી થાય છે?View Solution
- 5View Solutionનીચેનીમાંથી કયા ઘટકો ઇલેક્ટ્રોન અનુરાગી સ્વભાવમાં નથી?
- 6View Solutionઉપરોક્ત સંયોજનોના ઉષ્મા ના દહન નો યોગ્ય ક્રમ કયો છે ?
- 7$H - C \equiv C\mathop {-} \limits^a C \equiv C\mathop {-} \limits^b C{H_3}$View Solution
$a$ અને $b$ ની બંધ લંબાઈની તુલના કરો
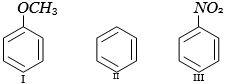
- 8નીચેના $(I-III)$ સંયોજનોમાં ઇલેક્ટ્રોન અનુરાગી પ્રક્રિયક સાથે પ્રક્રિયાનો યોગ્ય ક્રમ છે?View Solution
- 9View Solutionનીચેનામાંથી કયું એ અન્યનું સંસ્પંદન નથી
- 10નીચે આપેલા સંયોજનોમાં $pK_b$ નો વધતો ક્રમ શું હશે?View Solution