નીચેની માહિતી પરથી $1\, kg$ પાણીમાં $13.44\, g\, CuCl_2$ ઓગાળી બનાવેલા દ્રાવણના ઉત્કલનબિંદુમાં થતો વધારો ...... થશે.
$(M.wt.$ of $CuCl_2 =134.4 $ અને $K_b = 0.52\, , molal^{-1})$
IIT 2005, Medium
a
(a) $\Delta T_b = i.\,K_b\,.\,m$
(a) $\Delta T_b = i.\,K_b\,.\,m$
$CuCl_2$ $\to $ $Cu^{2+}$ + $2Cl^-$
$1$ $0$ $0$
$(1-\alpha$) $\alpha$ 2$\alpha$
$i = 1 + 2$ $\alpha$
Assuming $ 100\%$ ionization
So, $i = 3$
$T_b = 3$ $\times$ $0.52$ $\times$ $0.1 = 0.156 $ $\approx$ $0.16$
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionજ્યારે થોડીક માત્રામાં નેપ્થોલિનને બેન્ઝિનમાં ઉમેરવામાં આવે છે ત્યારે બેન્ઝિનના ઠારબિંદુમાં શું થાય છે ?
- 2જો $Na_2SO_4$ નો વિયોજન અંશ $\alpha$, વોન્ટ હોફ અવયવ $ (i)$ ની ગણતરી માટે કઈ રીતે વપરાય છે?View Solution
- 3$83\, {~g}$ ઇથિલિન ગ્લાયકોલ $625\, {~g}$ પાણીમાં ઓગળેલ છે. દ્રાવણનું ઠાર બિંદુ $......\, {K}$ છે. (નજીકના પૂર્ણાંકમાં)View Solution
[ઉપયોગ કરો: પાણીનો મોલલ અવનયન મંદન અચળાંક $\left.=1.86 \,{~K} \,{~kg} \,{~mol}^{-1}\right]$
પાણીનું ઠારબિંદુ $=273\, {~K}$
આણ્વિય દળ : ${C}: 12.0\, {u}, {O}: 16.0\, {u}, {H}: 1.0\, {u}]$
- 4View Solutionનીચેનામાંથી ક્યા મિશ્રણમાં મુખ્ય આંતરક્રિયા તરીકે દ્વિધુવ-દ્વિધ્રુવ બળ હાજર છે ?
- 5જો સમાન દ્રાવકમાં $5.25\% w/v$ પદાર્થનું દ્રાવણ $1.5\% w/v $ યુરિયાના દ્રાવણ સાથે આઈસોટોનીક થાય છે. (અ.ભા. $= 60\,g\,mol^{-1}$ ) તો પદાર્થનું અણુભાર ........ $g \, mol^{-1}$ હશે.View Solution
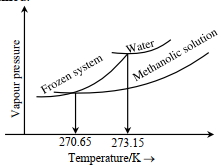
- 6જ્યારે'$x^{\prime} \times 10^{-2} \mathrm{~mL}$ મિથેનોલ (મોલર દળ=32 $\mathrm{g}$; ઘનતા $=0.792 \mathrm{~g} / \mathrm{cm}^3$ ) ને $100 \mathrm{~mL}$ પાણીમાં (ઘનતા $=1 \mathrm{~g} / \mathrm{cm}^3$ ), ઉમેરવામાં આવે છે ત્યારે નીચે મુજબ નો ડાયાગ્રામ પ્રાપ્ત થાય છે.View Solution
$(Image)$
$x=$.. . . . . .(નજીક નો પૂર્ણાક)
[આપેલ : $273.15 \mathrm{~K}$ પર પાણીનો મોલલ ઠારણ બિંદુ અવનયન અયળાંક $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ છે]
- 7$20^o$ સે. એ પાણીનું બાષ્પનું દબાણ $17.5$ મિમી $Hg$.છે. $20$ સે. એ $178.2$ ગ્રામ પાણીમાં $18 $ ગ્રામ ગ્લુકોઝ $(C_6H_{12}O_6)$ ઉમેરવામાં આવે છે પરિણામી દ્રાવણનું બાષ્પ દબાણ કેટલું હશે ?View Solution
- 8View Solution…… નું બાષ્પદબાણ મહત્તમ છે.
- 9નીચેનામાંથી કયા ક્ષારનું વોન્ટ હોફ અવયવ $i$નું મૂલ્ય ${K_4}[Fe{(CN)_6}]$ સાથે સમાન થશે.View Solution
- 10View Solutionસમઅભિસારી દ્રાવણો ......... સમાન ધરાવે છે.
